
Ethyl 4-(cyclopropylamino)-2-(methylthio)-pyrimidine-5-carboxylate synthesis
- Product Name:Ethyl 4-(cyclopropylamino)-2-(methylthio)-pyrimidine-5-carboxylate
- CAS Number:651734-65-5
- Molecular formula:C11H15N3O2S
- Molecular Weight:253.32
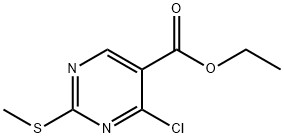
5909-24-0
404 suppliers
$6.00/5g
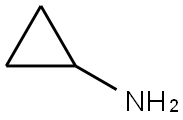
765-30-0
489 suppliers
$10.00/25g

651734-65-5
16 suppliers
$275.69/1gm:
Yield:651734-65-5 90%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20;
Steps:
2.A
Into an Argonaut Technologies' Quest 205 100 mL reactor was added 8.0 g (34.4 mmol) of 4-chloro-2-methylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester in 70 mL of dry THF. To the reaction mixture was added 1.1 equivalents of triethylamine followed by a solution of 1.1 equivalents of amine (R^NH2) i? 10 mL of dry THF. The reaction mixture was agitated overnight under a nitrogen atmosphere at room temperature. The THF solution was drained from the reaction vessel, and the residue was washed with THF. The THF solutions were combined, evaporated, and redissolved in 35 mL of ethyl acetate. The ethyl acetate solution was added back into the reaction vessel containing the residue, followed by 40 mL of 0.5 M NaOH. The reaction was agitated and the aqueous layer removed. The ethyl acetate solution was washed twice with 0.5 M NaOH EPO
References:
WO2006/38112,2006,A1 Location in patent:Page/Page column 30; 31

110-91-8
679 suppliers
$9.00/1g
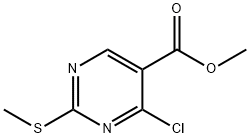
38275-39-7
13 suppliers
inquiry

651734-65-5
16 suppliers
$275.69/1gm: