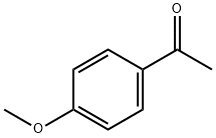
(E)-1-(4-methoxyphenyl)-3-(4-nitrophenyl)prop-2-en-1-one synthesis
- Product Name:(E)-1-(4-methoxyphenyl)-3-(4-nitrophenyl)prop-2-en-1-one
- CAS Number:6552-67-6
- Molecular formula:C16H13NO4
- Molecular Weight:283.28
Yield:6552-67-6 91%
Reaction Conditions:
with sodium hydroxide in ethanol;water at 20; for 12 h;Cooling with ice;
Steps:
1.2. General Procedure A: Synthesis of Nitrochalcone Derivatives
General procedure: 4-nitrobenzaldehyde (10 mmol) and the substituted acetophenone (10 mmol) was dissolved in 20 mL EtOH and dropwise 1N NaOH (20 mL) on ice bath. This solution was stirred for 12h at room temperature and neutralized with 1N HCl. The precipitated product was filtered and washed with a minimum amount of ethanol.
References:
Sonmez, Fatih;Sevmezler, Sedat;Atahan, Alparslan;Ceylan, Mustafa;Demir, Dudu;Gencer, Nahit;Arslan, Oktay;Kucukislamoglu, Mustafa [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 24,p. 7479 - 7482] Location in patent:supporting information; experimental part