Ethyl 5-(3-chlorophenyl)-[1,2,4]oxadiazole-3-carboxylate synthesis
- Product Name:Ethyl 5-(3-chlorophenyl)-[1,2,4]oxadiazole-3-carboxylate
- CAS Number:657424-68-5
- Molecular formula:C11H9ClN2O3
- Molecular Weight:252.65
Yield:657424-68-5 61.4%
Reaction Conditions:
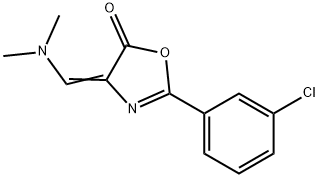
Stage #1: 2-(3-chloro-phenyl)-4-dimethylaminomethylene-4H-oxazol-5-one;ethanolwith sodium hydroxide for 0.5 h;Heating / reflux;
Stage #2: with hydrogenchloride;sodium nitrite in diethyl ether;water;
Steps:
67
2- (3-Chloro-phenyl)-4-dimethylaminomethylene-4H-oxazol-5-one (10.5, 41.9 mmole) was heated with sodium hydroxide (0.8 g, 20 mmol) in ethanol (120 mL) at reflux for 30 minutes. The reaction mixture was concentrated and the residue was mixed with 4% [HC1] (100 mL) and ether (100 mL). NaN02 (3.6 g, 52.2 mmol) in water [(20 ML)] was added dropwise. The reaction mixture was stirred vigorously overnight. The mixture was filtered through celite and washed with ether. The ether layer was washed with water and brine, concentrated, purified by column chromatography with dichloromethane to give 6.5 g (61.4 %) [OF 5- (3-CHLORO-PHENYL)- [1,] 2,4] [OXADIAZOLE-3-CARBOXYLIC] acid ethyl ester as pale-yellow [OIL.. LH-NMR (CDCL3) 6 (PPM)] : 8.26 (s, [1H),] 8.13 (d, [1H),] 7.64 (d, [1H),] 7. [53] (t, [1H),] 4. [58] (q, 2H) and 1.50 (t, 3H).
References:
WO2004/14370,2004,A2 Location in patent:Page/Page column 66
57728-59-3
51 suppliers
$35.35/250mgs:
![Ethyl 5-(3-chlorophenyl)-[1,2,4]oxadiazole-3-carboxylate](/CAS/GIF/657424-68-5.gif)
657424-68-5
14 suppliers
$94.25/1g