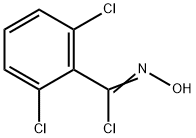
2,6-DICHLORO-N-HYDROXYBENZENECARBOXIMIDOYL CHLORIDE synthesis
- Product Name:2,6-DICHLORO-N-HYDROXYBENZENECARBOXIMIDOYL CHLORIDE
- CAS Number:6579-27-7
- Molecular formula:C7H4Cl3NO
- Molecular Weight:224.47

83-38-5
442 suppliers
$6.00/10g

6579-27-7
67 suppliers
$45.00/100mg
Yield: 73%
Reaction Conditions:
Stage #1:2,6-dichlorobenzaldehyde with hydroxylamine hydrochloride;potassium carbonate in ethanol;water at 0 - 90; for 2 h;
Stage #2: with N-chloro-succinimide in N,N-dimethyl-formamide at 0;
Steps:
1 Synthesis of Intermediate VI-1:
Add potassium carbonate aqueous solution (3N, 182 mmol) dropwise to a stirring solution of hydroxylamine hydrochloride (182 mmol) in ethanol (100 mL) at 0°C, 2,6-Dichlorobenzaldehyde (20g, 114mmol) is dissolved in 100ml ethanol, Then it was added to the hydroxylamine solution, the temperature was raised to 90°C, and the reaction was carried out for two hours. Wait for the mixture to cool to room temperature and then concentrate to a solid. Add water/ethanol (1000mL/100mL) solution and stir to break up the solid, filter, Vacuum drying was carried out at 50°C overnight to obtain the compound intermediate (18.4 g). This intermediate was dissolved in N,N-dimethylformamide (50mL), Add N-chlorosuccinimide (97mmol) in N,N-dimethylformamide (100mL) solution dropwise at 0°C, Stir overnight. Pour the reaction solution into ice water at 0°C, Then it was extracted with methyl tert-butyl ether (200mL each time, 3 times in total), The organic phase was washed with saturated brine and concentrated to obtain a crude product. Add n-hexane (600 mL) to the flask containing the crude product, stir with a magnet, and filter. The solid was dried under vacuum (30° C.) to obtain Intermediate III-1 (18.3 g, yield 73%).
References:
CN112824394, 2021, A Location in patent:Paragraph 0092-0096

25185-95-9
123 suppliers
$24.89/5g

6579-27-7
67 suppliers
$45.00/100mg