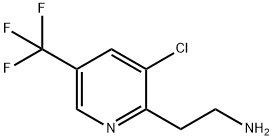
2-(3-chloro-5-(trifluoroMethyl)pyridin-2-yl)ethanaMine synthesis
- Product Name:2-(3-chloro-5-(trifluoroMethyl)pyridin-2-yl)ethanaMine
- CAS Number:658066-44-5
- Molecular formula:C8H8ClF3N2
- Molecular Weight:224.61
![2-[3-Chloro-5-(trifluoroMethyl)-2-pyridinyl]-acetonitrile](/CAS/GIF/157764-10-8.gif)
157764-10-8
58 suppliers
inquiry

658066-44-5
57 suppliers
inquiry
Yield: 76.59%
Reaction Conditions:
with hydrogen in tert-butyl methyl ether at 25; under 15001.5 Torr; for 4 h;Autoclave;
Steps:
1 Example 1: Hydrogenation with Raney cobalt catalyst
The water-comprising Raney cobalt catalyst (Actimet Cobalt (BASF)) is washed three times with water and another three times with tert- butyl methyl ether (MTBE). An autoclave is charged with 30 % (w/w) of the washed Raney cobalt catalyst and 66 g of [3-chloro-5- (trifluoromethyl)pyridin-2-yl]acetonitrile solved in 340 g of MTBE. Another 50 g of MTBE is added. The contents are then stirred at an elevated hydrogen pressure of 20 bar at 25 °C until the hydrogen uptake ceased after about three hours. Stirring is then continued for another hour. The reaction mixture is removed by filtration from the autoclave. The removed reaction mixture is analyzed by HPLC to quantify the content of amine. The product 2-[3-chloro-5-(trifluoromethyl)pyridin-2-yl]ethanamine was obtained at 76.59 % yield in a first example, and at 74.74 % in a second example.
References:
BAYER CROPSCIENCE AKTIENGESELLSCHAFT;MORADI, Wahed Ahmed;HIMMLER, Thomas;MÜLLER, Thomas Norbert WO2018/114810, 2018, A1 Location in patent:Page/Page column 14

69045-84-7
393 suppliers
$10.00/5g

658066-44-5
57 suppliers
inquiry
![Diethyl 2-[3-chloro-5-(trifluoromethyl)pyridin-2-yl]malonate](/StructureFile/ChemBookStructure22/GIF/CB3957884.gif)
172527-71-8
20 suppliers
$38.88/250mgs:

658066-44-5
57 suppliers
inquiry