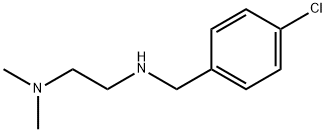
1,2-Ethanediamine, N'-[(4-chlorophenyl)methyl]-N,N-dimethyl- synthesis
- Product Name:1,2-Ethanediamine, N'-[(4-chlorophenyl)methyl]-N,N-dimethyl-
- CAS Number:65875-44-7
- Molecular formula:C11H17ClN2
- Molecular Weight:212.72
Yield:65875-44-7 66%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 20; for 16 h;
Steps:
Intermediate-3: Synthesis of N’-(4-chlorobenzyl)-N2,N2-dimethylethane- 1 ,2-diamine:
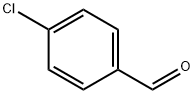
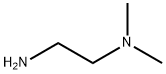
To a stirred solution of 4-chlorobenzaldehyde (15.0 g, 107.14 mmol, 1.0 eq) in methanol (250 ml) at 0 °C was added N’,N’-dimethylethane-1,2-diamine (11.65 ml, 107.14 mmol, 1.0 eq) and sodium borohydride (3.85 g, 107.14 mmol, 1.0 eq). The reaction mixturewas allowed to stir at room temperature for about 16 hours. After completion of the reaction (monitored by TLC), the reaction mixture was cooled to 0 °C, quenched with saturated ammonium chloride solution (20 ml) and extracted with ethyl acetate (3x200 ml). The combined organic extracts were washed with water (100 ml), dried over sodium sulfate,filtered and evaporated under reduced pressure to obtain the desired product (15.0 g, 66%yield) as a colourless liquid. ‘H NMR (300 MHz, DMSO-d6): ppm 7.32-7.23 (m, 4H), 3.76(s, 2H), 2.66 (t, J = 6.3 Hz, 2H), 2.41 (t, J = 6.3 Hz, 2H), 2.19 (s, 6H); ESI-MS: mlz 213.08(M+H)t
References:
WO2017/64628,2017,A1 Location in patent:Page/Page column 32; 33
![1,2-Ethanediamine, N2-[(4-chlorophenyl)methylene]-N1,N1-dimethyl-](/CAS/20210305/GIF/65875-11-8.gif)
65875-11-8
0 suppliers
inquiry
![1,2-Ethanediamine, N'-[(4-chlorophenyl)methyl]-N,N-dimethyl-](/CAS/20180531/GIF/65875-44-7.gif)
65875-44-7
6 suppliers
inquiry