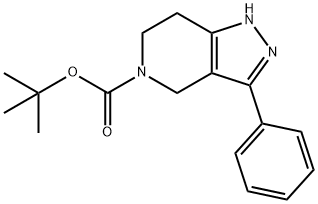
TERT-BUTYL 3-PHENYL-6,7-DIHYDRO-1H-PYRAZOLO[4,3-C]PYRIDINE-5(4H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-PHENYL-6,7-DIHYDRO-1H-PYRAZOLO[4,3-C]PYRIDINE-5(4H)-CARBOXYLATE
- CAS Number:661487-18-9
- Molecular formula:C17H21N3O2
- Molecular Weight:299.37
Yield:661487-18-9 90%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-onewith morpholine;toluene-4-sulfonic acid in toluene; for 16 h;Dean-Stark;Reflux;Inert atmosphere;
Stage #2: benzoyl chloridewith triethylamine in dichloromethane at 0 - 20; for 4 h;
Stage #3: with hydrazine in ethanol at 20; for 16 h;
Steps:
4.1.2 Preparation of tert-butyl 3-phenyl-6,7-dihydro-1H-pyrazolo[4,3-c]pyridine-5(4H)-carboxylate (3)
In a 100mL round-bottom flask equipped with a Dean-stark trap, a reflux condenser and an internal thermocouple, Compound 2 (1.0g, 4.63mmol), toluene (10mL), morpholine (0.42mL, 4.63mmol), and p-toluenesulfonic acid (catalytic) were added sequentially. The reaction solution was refluxed under N2 for 16h. The solvent was evaporated and the crude was dissolved in CH2Cl2 (20mL) and then triethylamine (1.07mL, 7.53mmol) was added at 0°C, under N2, a solution of benzoyl chloride (0.58mL, 5.02mmol) in CH2Cl2 (10mL) was added over 10min, the ice bath was then removed and the reaction solution was stirred at room temperature for 4h. All the volatile solvents were removed in vacuo and the residue was dissolved in ethanol, at 0°C, and hydrazine hydrate (0.25mL, 7.53mmol) was then added over 5min (exothermic reaction), the reaction solution was stirred at r.t. for 16h and the reaction mixture was concentrated under reduced pressure and the crude residue was purified by column chromatography to get compound 3 (1.34g, 90%) as an off-white solid. ESI-MS found 300 [M+H]+. 1H NMR (300MHz, CDCl3) δ 9.45 (s, 1H), 7.5-7.2 (m, 5H), 4.80 (s, 2H), 3.90 (t, J=7.8Hz, 2H), 2.81 (t, J=7.8Hz, 2H), 1.40 (s, 9H); 13C NMR (75MHz, CDCl3) δ 163.5, 147.6, 143.5, 136.6(2C), 132.7, 129.3(2C), 127.3, 117.5, 81.7, 43.9, 36.9, 31.5(3C), 27.7; Anal. calcd for C17H21N3O2: C, 68.20; H, 7.07; N, 14.04% Found: C, 68.24; H, 7.09; N, 14.13%.
References:
Samala, Ganesh;Devi, Parthiban Brindha;Nallangi, Radhika;Yogeeswari, Perumal;Sriram, Dharmarajan [European Journal of Medicinal Chemistry,2013,vol. 69,p. 356 - 364]

79099-07-3
543 suppliers
$5.00/5g
![TERT-BUTYL 3-PHENYL-6,7-DIHYDRO-1H-PYRAZOLO[4,3-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/661487-18-9.gif)
661487-18-9
17 suppliers
inquiry

98-88-4
575 suppliers
$10.00/5g
![TERT-BUTYL 3-PHENYL-6,7-DIHYDRO-1H-PYRAZOLO[4,3-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/661487-18-9.gif)
661487-18-9
17 suppliers
inquiry

24424-99-5
822 suppliers
$13.50/25G
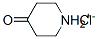
41979-39-9
118 suppliers
$15.00/10mg
![TERT-BUTYL 3-PHENYL-6,7-DIHYDRO-1H-PYRAZOLO[4,3-C]PYRIDINE-5(4H)-CARBOXYLATE](/CAS/GIF/661487-18-9.gif)
661487-18-9
17 suppliers
inquiry