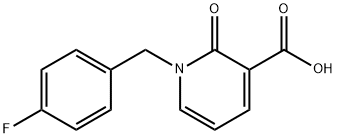
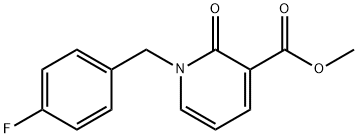
1-(4-fluorobenzyl)-1,2-dihydro-2-oxopyridine-3-carboxylic acid synthesis
- Product Name:1-(4-fluorobenzyl)-1,2-dihydro-2-oxopyridine-3-carboxylic acid
- CAS Number:66158-41-6
- Molecular formula:C13H10FNO3
- Molecular Weight:247.22
Yield:66158-41-6 96%
Reaction Conditions:
Stage #1: 2-hydroxy-3-carboxypyridinewith sodium hydride in N,N-dimethyl-formamide at 20; for 1 h;
Stage #2: 1-chloromethyl-4-fluorobenzene in N,N-dimethyl-formamide at 50; for 12 h;
Stage #3: with sodium hydroxide in water; for 4 h;Reflux;
Steps:
8 4.1.8. 1,2-Dihydro-1-(4-fluorobenzyl)-2-oxo-pyridine-3-carboxylic acid (12)
Commercial available 2-hydroxynicotinic acid (1.5 ?g, 10.78? mmol) was dissolved in anhydrous DMF (20.0 ?mL) and then NaH (650.0 ?mg, 27.08 ?mmol) was slowly added. After 1?h?at room temperature, p-fluorobenzyl chloride (1.5 mL, 12.52?mmol) was added and the reaction mixture was heated under stirring at 50?°C for 12?h. After cooling to room temperature, the reaction mixture was evaporated under reduced pressure affording a white solid that was treated with a solution of 10% NaOH (20.0? mL) and heated at reflux for 4?h. Then the mixture was cooled at room temperature and acidified with concentrated HCl until pH?=?1-2. The obtained solid was filtered under vacuum, washed with water and dried to afford pure compound 12 (2.6 ?g, yield: 96%). 1H NMR (400MHz, DMSO): δ 12.02 (bs, 1H, COOH), 8.42-8.47 (m, 2H, H4 and H6 Py) 7.22-7.50 (m, 4H, ArH), 6.83-6.80 (m, 1H, H5 Py), 5.34 (s, 2H, N-CH2).
References:
Chicca, Andrea;Arena, Chiara;Bertini, Simone;Gado, Francesca;Ciaglia, Elena;Abate, Mario;Digiacomo, Maria;Lapillo, Margherita;Poli, Giulio;Bifulco, Maurizio;Macchia, Marco;Tuccinardi, Tiziano;Gertsch, Jürg;Manera, Clementina [European Journal of Medicinal Chemistry,2018,vol. 154,p. 155 - 171]
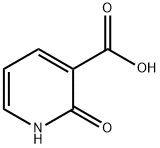
10128-91-3
109 suppliers
$9.00/250mg
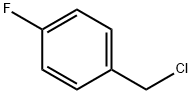
352-11-4
372 suppliers
$5.00/25g

66158-41-6
9 suppliers
inquiry
888721-12-8
1 suppliers
inquiry

66158-41-6
9 suppliers
inquiry