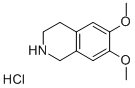
2(1H)-Isoquinolinebutanamine, 3,4-dihydro-6,7-dimethoxy- synthesis
- Product Name:2(1H)-Isoquinolinebutanamine, 3,4-dihydro-6,7-dimethoxy-
- CAS Number:669067-80-5
- Molecular formula:C15H24N2O2
- Molecular Weight:264.36
Yield:669067-80-5 93%
Reaction Conditions:
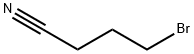
Stage #1: 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline monohydrochloride;4-bromobutanenitrilewith triethylamine in chloroform;Reflux;
Stage #2: with lithium aluminium tetrahydride in tetrahydrofuran at 0;Reflux;
Steps:
1.4 Synthesis of intermediate 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-2-butylamine
Take 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride 1.0g (4.35mmol),0.71 g (4.79 mmol) of 4-bromo-1-butyronitrile and 1.5 g (10.86 mmol) of triethylamine were dissolved in 15 ml of chloroform, and the mixture was heated and refluxed overnight for reaction. After the reaction is over, pour the reaction solution into 50ml of water,The aqueous layer was extracted with 3×50ml dichloromethane, the organic phase was collected and dried with anhydrous sodium sulfate,2.5g silica gel powder was added, and the product was purified by silica gel column chromatography (eluent: ethyl acetate:Petroleum ether = 1:1) white solid 0.9g, the yield is 80%.Dissolve 0.55g of the solid in 25ml THF, take an ice bath to 0°C, add 1.5g (39.53mmol) of LiAlH4 in batches, slowly heat up to reflux and react overnight.TLC monitors the completion of the reaction, adds ice water to quench the reaction, adds anhydrous sodium sulfate, and filters with suction.Extract with dichloromethane (3×50ml), collect the organic phase,It was spin-dried to obtain 0.3 g of a yellow oily product, which was directly used in the next step without purification.
References:
CN112898278,2021,A Location in patent:Paragraph 0070; 0075-0077; 0083-0085
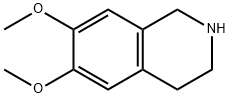
1745-07-9
114 suppliers
$8.00/250mg

669067-80-5
5 suppliers
inquiry
1207842-90-7
1 suppliers
inquiry

669067-80-5
5 suppliers
inquiry
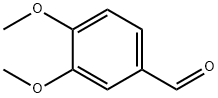
120-14-9
755 suppliers
$5.00/25g

669067-80-5
5 suppliers
inquiry
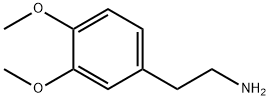
120-20-7
542 suppliers
$5.00/5G

669067-80-5
5 suppliers
inquiry