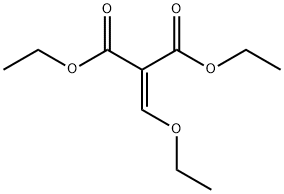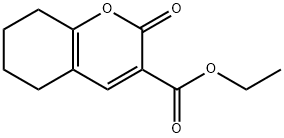
Ethyl 2-oxo-5,6,7,8-tetrahydro-2H-chromene-3-carboxylate synthesis
- Product Name:Ethyl 2-oxo-5,6,7,8-tetrahydro-2H-chromene-3-carboxylate
- CAS Number:66979-47-3
- Molecular formula:C12H14O4
- Molecular Weight:222.24
Yield:66979-47-3 50%
Reaction Conditions:
Stage #1: cyclohexanonewith lithium diisopropyl amide in tetrahydrofuran at -78 - -5; for 2 h;
Stage #2: diethyl 2-ethoxymethylenemalonate in tetrahydrofuran at -30 - 25; for 16 h;
Steps:
24
A solution of cyclohexanone (5 g, 1 eq.) in 60 mL of dry THF was added dropwise (over a period of 30 minutes) to a -78°C solution of freshly generated lithium diisopropylamide (31 mL, 1.2 eq.) in 75 mL of dry THF under dry N2. The resulting mixture was stirred at -78°C to -5°C over 2 hours. After the solution was cooled to -300C, diethyl ethoxymethylene malonate (12.4 mL, 1.2 eq.) in 20 mL of THF was added slowly (over a period of 15 ~ 20 minutes). The mixture was stirred at -300C to 25°C for 16 hours. The solution was then poured onto 100 mL of 5% aqueous HCl and extracted with CH2CI2 (5x30 mL). The combined extracts were dried over Na2SO4 and concentrated under vacuum. The crude product thus obtained was purified by silica gel column chromatography to give compound 1-28 as a white solid (5.7 g, 50%). 1H NMR (CDCl3) δ.7.98 (s, IH), 4.35 (q, J = 6.9 Hz, 2H), 2.60- 2.55 (m, 2H), 2.48-2.44 (m, 2H), 1.84-1.74 (m, 4H), 1.36 (t, J = 7.2 Hz, 3H). ESI-MS (M+H+) = 223.
References:
WO2008/95058,2008,A1 Location in patent:Page/Page column 37