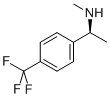
(S)-N-METHYL-1-[4-(TRIFLUOROMETHYL)PHENYL]ETHYLAMINE synthesis
- Product Name:(S)-N-METHYL-1-[4-(TRIFLUOROMETHYL)PHENYL]ETHYLAMINE
- CAS Number:672906-71-7
- Molecular formula:C10H12F3N
- Molecular Weight:203.2
![Benzenemethanamine, N,α-dimethyl-N-[(1S)-1-phenylethyl]-4-(trifluoromethyl)-, (αS)-](/CAS/20210305/GIF/672906-92-2.gif)
672906-92-2
0 suppliers
inquiry
![(S)-N-METHYL-1-[4-(TRIFLUOROMETHYL)PHENYL]ETHYLAMINE](/CAS/GIF/672906-71-7.gif)
672906-71-7
16 suppliers
inquiry
Yield:672906-71-7 94%
Reaction Conditions:
Stage #1: C18H20F3Nwith hydrogen;acetic acid;5%-palladium/activated carbon in methanol;water at 60; under 3750.38 Torr; for 13.5 h;
Stage #2: with sodium hydroxide in water;
Steps:
5.b Step (b), hydrogenolysis.
Step (b), hydrogenolysis. To 1.6ml of methanol, 0.50g (l.63mmol, 1eq.) of the above purified product of the optically active tertiary amine, 0.49g (8.16mmol, 5.O1eq.) of acetic acid, and 24.9mg (0.125 wt% Pd) of a palladium catalyst (having 5% palladium carried on an activated carbon containing 50 wt% of water) were added. The hydrogen pressure was adjusted to O.SMPa, and the stirring was conducted at 60°C for 13.5hr. After the reaction, the reaction liquid was filtrated using a filtration aid (CELITE (trade name)), concentrated, and vacuum-dried, thereby obtaining a crude product of an acetate of an optically active (S)-1-(4-trifluoromethylphenyl)ethylamine N-monomethyl, represented by the following formula. Then, 1N sodium hydroxide aqueous solution was added to the above crude product of the acetate to have a basic solution, followed by extraction with ethyl acetate. The recovered organic layer was dried with anhydrous sodium sulfate, followed by filtration, concentration, and vacuum drying, thereby obtaining 0.31g of a crude product of an optically active (S)-l-(4-trifluoromethylphenyl)ethylamine N-monomethyl represented by the following formula. The yield was 94%. The above crude product was found by chiral gas chromatography to have a conversion of 100% and an enantiomeric excess of 99.1 %ee. In terms of severing position selectivity whether the N-C* bond is severed at the broken line "a" to produce a compound A or at the broken line "b" to produce a compound B (i.e., the above optically active (S)-1-(4-trifluoromethylphenyl)ethylamine N-monomethyl) in the above formula 11, the above crude product was found by chiral gas chromatography to contain 1 part by mole of the compound A and 100 parts by mole of the compound B. 1H-NMR(TMS, CDCl3), δppm: 1.51 (d, 6.8 Hz, 3H), 2.35 (s, 3H), 3.88 (q, 6.8 Hz, 1H), 5.16 (br, 1H), 7.54 (Ar-H, 2H), 7.63 (Ar-H, 2H).
References:
WO2004/22521,2004,A1 Location in patent:Page 42-43
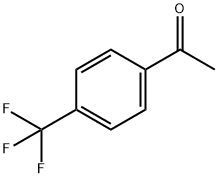
709-63-7
255 suppliers
$5.00/1g
![(S)-N-METHYL-1-[4-(TRIFLUOROMETHYL)PHENYL]ETHYLAMINE](/CAS/GIF/672906-71-7.gif)
672906-71-7
16 suppliers
inquiry