
α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate synthesis
- Product Name:α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate
- CAS Number:6736-63-6
- Molecular formula:C14H17BrF3NO8
- Molecular Weight:464.19
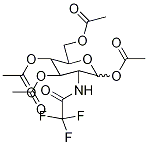
7139-63-1
16 suppliers
$125.00/250mg
![α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate](/CAS/20211123/GIF/6736-63-6.gif)
6736-63-6
2 suppliers
inquiry
Yield:6736-63-6 85%
Reaction Conditions:
with hydrogen bromide;acetic acid in dichloromethane at 20; for 16 h;Cooling with ice;Inert atmosphere;
Steps:
Synthesis of Compound 51
β-D-glucopyranose, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 1,3,4,6-tetraacetate (0.57 g, 1.28 mmol) was dissolved in CH2Cl2 (30 mL), and 0.69 M HBr in AcOH (6 mL) was slowly added dropwise with ice cooling under an Ar atmosphere. After the completion of the dropwise addition, the reaction solution was returned to room temperature and stirred. After 16 hours, the disappearance of the raw material was confirmed by TLC (hexane:EtOAc=1:2, 5% sulfuric acid in MeOH). The reaction solution was cooled with ice, brine was added, and the mixture was stirred. After 30 minutes, the aqueous layer was removed, saturated sodium bicarbonate water was added, and the mixture was stirred. The reaction solution was diluted with CH2Cl2 and then subjected to liquid-liquid extraction with a saturated aqueous sodium hydrogen carbonate solution and water. After washed with brine, the organic layer was dried over anhydrous sodium sulfate and distilled under reduced pressure. A compound 51 (0.51 g, 1.09 mmol, 85%) was obtained as an oil. (0183) 1H NMR (400 MHz, CDCl3) δ6.7-6.68 (d, J=9.8 Hz, 1H), 6.55-6.54 (d, J=5.4 Hz, 1H), 5.42-5.36 (t, J=10.2 Hz, 1H), 5.31-5.26 (t, J=9.8 Hz, 1H), 4.36-4.32 (m, 2H), 4.28-4.25 (m, 1H), 4.16-4.13 (d, J=12.7 Hz, 1H), 2.12 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H).
References:
US2018/94017,2018,A1 Location in patent:Paragraph 0182; 0183
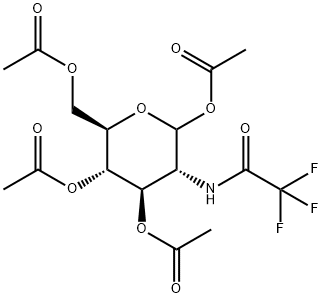
137766-83-7
6 suppliers
inquiry
![α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate](/CAS/20211123/GIF/6736-63-6.gif)
6736-63-6
2 suppliers
inquiry

7597-81-1
35 suppliers
$14.00/250mg
![α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate](/CAS/20211123/GIF/6736-63-6.gif)
6736-63-6
2 suppliers
inquiry
![6-(hydroxymethyl)-3-[(4-methoxyphenyl)methylideneamino]oxane-2,4,5-tri ol](/CAS/GIF/51471-40-0.gif)
51471-40-0
34 suppliers
$28.60/50MG
![α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate](/CAS/20211123/GIF/6736-63-6.gif)
6736-63-6
2 suppliers
inquiry
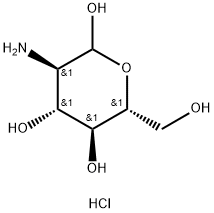
1078691-95-8
9 suppliers
inquiry
![α-D-Glucopyranosyl bromide, 2-deoxy-2-[(2,2,2-trifluoroacetyl)amino]-, 3,4,6-triacetate](/CAS/20211123/GIF/6736-63-6.gif)
6736-63-6
2 suppliers
inquiry