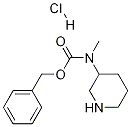
PIPERIDIN-3-YLMETHYL-CARBAMIC ACID BENZYL ESTER-HCl synthesis
- Product Name:PIPERIDIN-3-YLMETHYL-CARBAMIC ACID BENZYL ESTER-HCl
- CAS Number:676621-99-1
- Molecular formula:C14H21ClN2O2
- Molecular Weight:284.7817
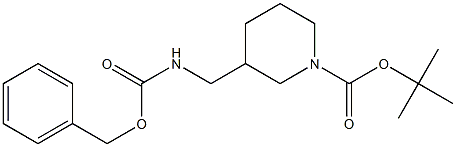
478366-01-7
0 suppliers
inquiry

676621-99-1
13 suppliers
inquiry
Yield:676621-99-1 84%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 0; for 1.33333 h;
Steps:
4.B
Part B: Preparation of 3-(Benzyloxycarbonylaminomethyl)-piperidine Hydrochloride A solution of 3-(benzyloxycarbonylaminomethyl)-piperidine-1-carboxylic acid tert-butyl ester (594 mg, 1.7 mmol) in ethyl acetate (10 mL) was stirred on an ice bath and treated with a solution of hydrogen chloride in dioxane (4.0 N, 10 mL, 40 mmol). The mixture was stirred for 80 minutes, then was concentrated under vacuum. The gummy residue was triturated repeatedly in ether to provide a white powder (408 mg, 84%)which was extremely hygroscopic and was used without further purification. 1H NMR (300 MHz, CD3OD) δ 7.34 (m, 5H), 5.07 (s, 2H), 3.34 (m, 2H), 3.09 (m, 2H), 2.88 (m, 1H), 2.66 (m, 1H), 1.90 (m, 3H), 1.70 (m, 1H), 1.30 (m, 1H).
References:
US2004/67935,2004,A1 Location in patent:Page/Page column 31
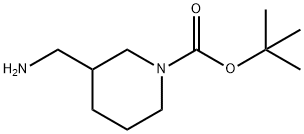
162167-97-7
210 suppliers
$10.00/250mg

676621-99-1
13 suppliers
inquiry

501-53-1
392 suppliers
$10.00/1g

676621-99-1
13 suppliers
inquiry