
2',3'-Dihydro-4'H-spiro[cyclopropane-1,1'-naphthalen]-4'-one synthesis
- Product Name:2',3'-Dihydro-4'H-spiro[cyclopropane-1,1'-naphthalen]-4'-one
- CAS Number:67688-27-1
- Molecular formula:C12H12O
- Molecular Weight:172.22
![Spiro[cyclopropane-1,1'(2'H)-naphthalene], 3',4'-dihydro-](/CAS/20210305/GIF/25033-23-2.gif)
25033-23-2
2 suppliers
inquiry
![2',3'-Dihydro-4'H-spiro[cyclopropane-1,1'-naphthalen]-4'-one](/CAS/20210111/GIF/67688-27-1.gif)
67688-27-1
6 suppliers
inquiry
Yield:67688-27-1 77%
Reaction Conditions:
with tert.-butylhydroperoxide;tetrakis(μ-caprolactamato)dirhodium(Rh-Rh);sodium hydrogencarbonate in 1,2-dichloro-ethane;Heating;
Steps:
1.9.C Step C: Preparation of 2'H-spiro[cyclopropane-l,l'-naphthalen]-4'(3'/ )-one
Step C: Preparation of 2'H-spiro[cyclopropane-l,l'-naphthalen]-4'(3'/ )-one To a mixture of 3',4'-dihydro-2' /-spiro[cyclopropane-l,r-naphthalene] (861 mg, 5.441 mmol), sodium bicarbonate (460 mg, 5.476 mmol), and Rh2(cap)4 (29.6 mg, 69.94 μιηο) in 20 mL DCE, 5.5 M 2-hydroperoxy-2-methylpropane in decane (5 mL, 27.50 mmol) was added. The mixture was stirred at 40°C (oil bath). After 3 h, more Rh2(cap)4 (25 mg) was added. After stirring overnight, the mixture was extracted with water and CH2CI2. Organic phases were dried over MgS04, filtered, and concentrated. The residue was purified by biotage column chromatography (S1O2, hexane/ AcOEt gradient) to give 2' /-spiro[cyclopropane-l,r-naphthalen]-4'(3' )-one (718 mg, 77 %) as a colorless oil. NMR (400 MHz, CDCI3) δ 0.97-1.00 (m, 2H), 1.09-1.11 (m, 2H), 1.97-2.00 (m, 2H), 2.76-2.79 (m, 2H), 6.82 (dd, J, = 8.0 Hz, J2 = 0.64 Hz, 1H), 7.23-7.27 (m, 1H), 7.43-7.47 (m, 1H), 8.03 (dd, J, = 7.8 Hz, J2 = 1.3 Hz, 1H).
References:
WO2017/23679,2017,A1 Location in patent:Page/Page column 118-119
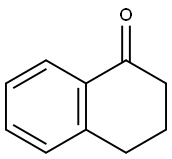
529-34-0
478 suppliers
$6.00/10g
![2',3'-Dihydro-4'H-spiro[cyclopropane-1,1'-naphthalen]-4'-one](/CAS/20210111/GIF/67688-27-1.gif)
67688-27-1
6 suppliers
inquiry
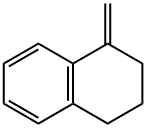
25108-63-8
1 suppliers
inquiry
![2',3'-Dihydro-4'H-spiro[cyclopropane-1,1'-naphthalen]-4'-one](/CAS/20210111/GIF/67688-27-1.gif)
67688-27-1
6 suppliers
inquiry