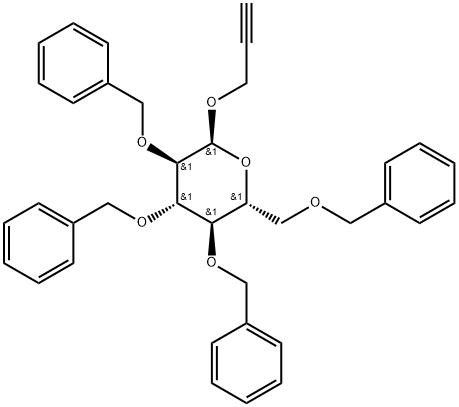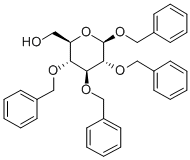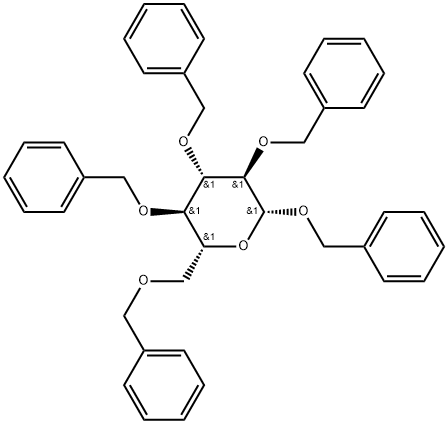
β-D-Glucopyranoside, phenylmethyl 2,3,4,6-tetrakis-O-(phenylmethyl)- synthesis
- Product Name:β-D-Glucopyranoside, phenylmethyl 2,3,4,6-tetrakis-O-(phenylmethyl)-
- CAS Number:67890-29-3
- Molecular formula:C41H42O6
- Molecular Weight:630.78
Yield:67890-29-3 96%
Reaction Conditions:
with FeCl3/C in acetonitrile at 60; for 3 h;Mechanism;Inert atmosphere;Green chemistry;stereoselective reaction;Reagent/catalyst;Temperature;
Steps:
Reaction of 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside with Alcohols and Phenols in the Presence of FeCl3/C; General Procedure
General procedure: A suspension of the FeCl3/C catalyst (2 mg, 0.002 mmol) in anhydCH3CN (6 mL) was prepared in a round-bottomed flask. A portion ofthis preparation (1.5 mL) was added to a mixture of required 2,3,4,6-tetra-O-benzyl-α-D-glucopyranoside (58 mg, 0.1 mmol) and the respective acceptor (0.12 mmol) in a round-bottomed flask (5 mL) under N2 atmosphere and the reaction mixture was stirred at 60 °C. After completion of the reaction (monitored by TLC), the organic phase was condensed under vacuum to obtain the crude product, whichwas purified by silica gel column chromatography (PE/EtOAc 40:1). All new compounds were fully characterized by 1H NMR, 13C NMR,and MS. Spectral and analytical data were in good agreement with the desired structures.
References:
Guo, Hong;Li, Juan;Si, Wenshuai;Tang, Jie;Tang, Tianjun;Wang, Zhongfu;Yang, Guofang;Zhang, Jianbo [Synthesis,2019,vol. 51,# 15,p. 2984 - 3000]
74808-09-6
66 suppliers
$32.00/100mg

100-51-6
1383 suppliers
$5.00/100g

67890-29-3
1 suppliers
inquiry