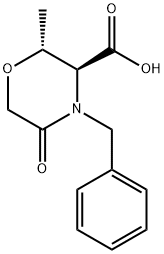
(2R,3S)-2-Methyl-5-oxo-4-(phenylMethyl)-3-Morpholinecarboxylic acid synthesis
- Product Name:(2R,3S)-2-Methyl-5-oxo-4-(phenylMethyl)-3-Morpholinecarboxylic acid
- CAS Number:681851-25-2
- Molecular formula:C13H15NO4
- Molecular Weight:249.26
Yield:681851-25-2 71%
Reaction Conditions:
Stage #1: (2S,3R)-2-(benzylamino)-3-hydroxybutanoic acid;chloroacetyl chloridewith potassium carbonate in tetrahydrofuran;water at 0;
Stage #2: with sodium hydroxide in tetrahydrofuran;water at 5; for 4 h;
Steps:
5.2 Step 2) (2R, 3S) -4-benzyl-2-methyl-5-oxomorpholine-3-carboxylic acid
To a mixture of (2S, 3R) -2- (benzylamino) -3-hydroxybutanoic acid (21.4 g, 102.4 mmol) , THF (110 mL) and a solution of K2CO3 (42.5 g, 307.2 mmol) in water (70 mL) was added 2-chloroacetyl chloride (17.8 g, 157.7 mmol) slowly at 0 . After the addition, the mixture was stirred at 0 for 3 hours, then to the mixture was added a solution of sodium hydroxide (16.4 g, 409.6 mmol) in water (40 mL) . After the addition, the mixture was stirred at 5 for 4 hours, the mixture was warmed to 25 . The resulting mixture was extracted with PE (50 mL×2) . The separated water phase was cooled to 15 , and to the water phase was added concentrated hydrochloric acid until a lot of solid precipitate was formed. The mixture was stirred at 10 for 12 hours, then filtered. The filter cake was washed with water to give the title compound as a white solid (18.1 g, 71) . The compound was characterized by the following spectroscopic data: [0373] MS (ESI, pos. ion) m/z: 250.1 [M+H] +.
References:
WO2015/144093,2015,A1 Location in patent:Paragraph 00174

100-52-7
973 suppliers
$20.37/250g

681851-25-2
8 suppliers
inquiry

