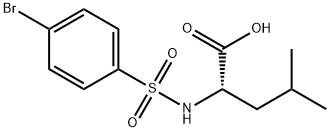
2-([(4-BROMOPHENYL)SULFONYL]AMINO)-4-METHYLPENTANOIC ACID synthesis
- Product Name:2-([(4-BROMOPHENYL)SULFONYL]AMINO)-4-METHYLPENTANOIC ACID
- CAS Number:68305-78-2
- Molecular formula:C12H16BrNO4S
- Molecular Weight:350.23
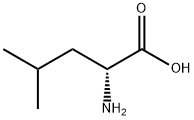
328-38-1
431 suppliers
$5.00/5g

98-58-8
248 suppliers
$12.00/5g
![2-([(4-BROMOPHENYL)SULFONYL]AMINO)-4-METHYLPENTANOIC ACID](/CAS/GIF/68305-78-2.gif)
68305-78-2
16 suppliers
$75.00/100mg
Yield:68305-78-2 96.4%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;
Steps:
56.a a)
a)
Preparation of N-p-bromo-benzenesulfonyl-D-leucine
D-leucine (1.4 g) was dissolved in 1.5N sodium hydroxide solution (10 ml), dioxane (10 ml) was added, and cooled down to control the temperature between 0 and 5° C.; p-bromobenzenesulfonyl chloride (2.5 g) and 1.5N sodium hydroxide solution were added slowly dropwisely to maintain the pH at 9-10; the resulting mixture was allowed to react at 0° C. for 2 h and warm up naturally to room temperature to react for 2 h.
Upon cooled, dilute hydrochloric acid was added dropwisely to adjust pH to 3, and the resulting mixture was concentrated under reduced pressure to remove dioxane, and the water phase was extracted with ethyl acetate (20 ml*3), the resulting organic phase was washed with acid, base and water until to be neutral, dried over anhydrous sodium sulfate, and the filtrate was concentrated and purified on a column to give a white solid (2 g, 96.4%).
References:
US2013/296245,2013,A1 Location in patent:Page/Page column