
2-(1-NAPHTHYLENE)-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE synthesis
- Product Name:2-(1-NAPHTHYLENE)-4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLANE
- CAS Number:68716-52-9
- Molecular formula:C16H19BO2
- Molecular Weight:254.13
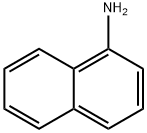
134-32-7
360 suppliers
$9.00/1g

73183-34-3
562 suppliers
$6.00/5g

68716-52-9
128 suppliers
$7.00/250mg
Yield: 85%
Reaction Conditions:
Stage #1:1-amino-naphthalene with hydrogenchloride;methanol;sodium nitrite in water at 0 - 5; for 0.5 h;Green chemistry;Sandmeyer Reaction;
Stage #2:bis(pinacol)diborane in water at 20; for 1 h;Green chemistry;Sandmeyer Reaction;
Steps:
General Procedure for the Synthesis of Aryboronate Esters
General procedure: To a solution of arylamine (0.5 mmol, 1.0 equiv) in MeOH(1.0 mL) was added HCl (0.5 mL, 1.5 mmol, 3.0 equiv) followed by H2O (0.5 ml). This mixture was stirred 2 min, and the NaNO2 solution (0.25 mL) was then added. The NaNO2 solution was prepared by dissolving 35 mg of NaNO2 in H2O (0.25 mL). This mixture was stirred 30 minat 0-5 °C followed by B2pin2 (2, 381 mg, 1.5 mmol, 3.0equiv) in MeOH (1.0 mL). This mixture was stirred 60 min.H2O (10 mL) was added to the reaction mixture, then extracted with CH2Cl2 (50 mL, 3×). The combined organic layers were washed with sat. NaHCO3, dried over Na2SO4, followed by evaporation, and the crude residue was purified by flash chromatography.
References:
Zhao, Cong-Jun;Xue, Dong;Jia, Zhi-Hui;Wang, Chao;Xiao, Jianliang [Synlett,2014,vol. 25,# 11,art. no. ST-2014-W0180-L,p. 1577 - 1584] Location in patent:supporting information
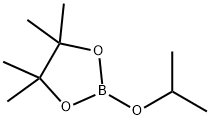
61676-62-8
310 suppliers
$10.00/5g
90-11-9
503 suppliers
$5.00/10g

68716-52-9
128 suppliers
$7.00/250mg

73183-34-3
562 suppliers
$6.00/5g
90-13-1
196 suppliers
$17.00/25G

68716-52-9
128 suppliers
$7.00/250mg
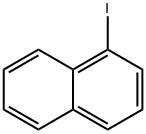
90-14-2
284 suppliers
$19.00/5g

73183-34-3
562 suppliers
$6.00/5g

68716-52-9
128 suppliers
$7.00/250mg

90-11-9
503 suppliers
$5.00/10g

73183-34-3
562 suppliers
$6.00/5g

68716-52-9
128 suppliers
$7.00/250mg