
2,6,10,14,18,22,26,30,34,38-Tetracontadecaene, 1-bromo-3,7,11,15,19,23,27,31,35,39-decamethyl-, (2E,6E,10E,14E,18E,22E,26E,30E,34E)- synthesis
- Product Name:2,6,10,14,18,22,26,30,34,38-Tetracontadecaene, 1-bromo-3,7,11,15,19,23,27,31,35,39-decamethyl-, (2E,6E,10E,14E,18E,22E,26E,30E,34E)-
- CAS Number:68799-83-7
- Molecular formula:C50H81Br
- Molecular Weight:762.08

15575-04-9
8 suppliers
inquiry

68799-83-7
0 suppliers
inquiry
Yield:68799-83-7 39.5 g (90.4%)
Reaction Conditions:
with pyridine;phosphorus tribromide in hexane;
Steps:
4.a a
a 1-Bromo-3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaene Under an atmosphere of argon, a solution of phosphorus tribromide (8.32 g, 30.7 mmol) in 27 ml of n-hexane was added in dropwise to a mixture of 3,7,11,15,19,23,27,31,35,39-decamethyl-2,6,10,14,18,22,26,30,34,38-tetracontadecaen-1-ol (40 g, 57.3 mmol) and pyridine (1.92 ml, 23.8 mmol) in 587 ml of n-hexane at the temperature below 10° C. The solution was stirred at 10° C. for an hour, allowed to warm slowly from 10° to 20° C., stirred at 20°-25° C. for an hour and poured into cracked ice with stirring. The resulting precipitate was filtered off and the filtrate was extracted with n-hexane. The organic layer was washed with 5% sodium bicarbonate aqueous solution, dried over anhydrous sodium sulfate, filtered and evaporated in vacuo to dryness to afford 39.5 g (90.4%) of the desired compound, as a yellow oil. TLC: Rf=0.34 (silica gel, n-hexane:AcOEt=5:1). IR spectrum (vmaxneat) cm-1: 2980, 2940, 1670. 1 H-NMR spectrum (CDCl3) δ ppm: 1.60-2.15 (69H, m, --CH3 *11, --CH2 --*18), 4.03 (2H, d, J=8.3 Hz, --CH2 Br), 5.09-5.14 (9H, m, =CHCH2 CH2 --*9), 5.53 (1H, t, J=8.3 Hz, =CHCH2 Br).
References:
US5177102,1993,A

59640-02-7
0 suppliers
inquiry

68799-83-7
0 suppliers
inquiry

13190-97-1
348 suppliers
$30.00/20mg

68799-83-7
0 suppliers
inquiry

52610-77-2
10 suppliers
$80.00/10mg

68799-83-7
0 suppliers
inquiry
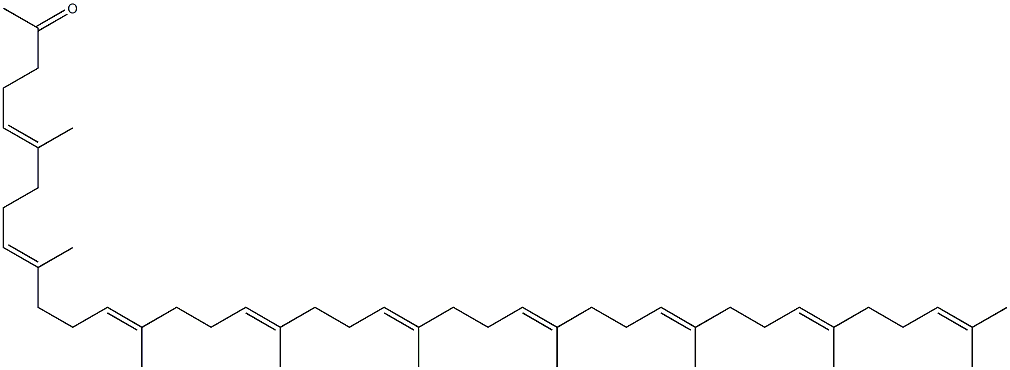
65717-26-2
3 suppliers
inquiry

68799-83-7
0 suppliers
inquiry