
1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID synthesis
- Product Name:1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID
- CAS Number:692281-53-1
- Molecular formula:C11H13N3O2
- Molecular Weight:219.24
![methyl 2-[3-(4-methoxyphenyl)propanoylamino]benzoate](/CAS/20180720/GIF/83988-43-6.gif)
83988-43-6
1 suppliers
inquiry
![1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/StructureFile/ChemBookStructure8/GIF/CB1119552.gif)
692281-53-1
10 suppliers
inquiry
Yield:692281-53-1 63%
Reaction Conditions:
Stage #1: methyl N-<3-(4-methoxyphenyl)propanoyl>anthranilatewith lithium hydroxide monohydrate in tetrahydrofuran;methanol at 20;
Stage #2: with hydrogenchloride in water; pH=4;
Steps:
Approach 1: Synthesis of N-cinnamoylanthranilate derivatives from cinnamic acid derivatives
General procedure: Cinnamoyl chlorides - Derivatives of cinnamic acid were stirred with oxalyl chloride (5 ml g-1) for up to 4 h. The stirring time differed depending on the dissolution rate of the starting cinnamic acid derivative. For derivatives with 4-NO2 or 4-N(CH3)2 substituents, a drop of dry N,N-DMF was added to catalyze the reaction. The excess reagent was evaporated under reduced pressure. The hydroxy groups in the hydroxycinnamic acid derivatives were acetylated prior to reaction with oxalyl chloride by stirring the derivative (0.0244 mol) in acetic anhydride (5 ml g-1) and pyridine (0.5 ml) at room temperature (rt, ~20 °C) overnight. Cold water (~50 ml) was added to the mixture and stirred for further 5-10 min with cooling in an ice-water bath, and the resulting precipitate of acetoxycinnamic acid was obtained by vacuum filtration, washed with cold water and dried.Methyl N-cinnamoylanthranilates - The cinnamoyl chloride (0.014 mol) was added to a solution of excess methyl anthranilate (0.017 mol) in dry pyridine (10 ml g-1), and the mixture was stirred for an hour at rt. Cold water (250 ml) was added to the reaction mixture and the resulting precipitate was filtered and washed with cold water until free of pyridine, and was recrystallized from hot ethanol. In contrast, methyl N-hydrocinnamoylanthranilate derivatives, with a low melting point, were extracted in diethyl ether (2 × 70 ml), washed with cold water (2 × 50 ml) and the solvent evaporated under reduced pressure.N-Cinnamoylanthranilic acids - The methyl N-cinnamoylanthranilate (0.010 mol) was stirred in a mixture of THF (100 ml) and methanol (20 ml), and LiOH.H2O (0.050 mol) [LiOH dissolved in water 0.2 g per 10 ml] was added to the reaction mixture and stirred at rt overnight. The excess reagent and solvents were evaporated under reduced pressure. The crude product was dissolved in water (~350 ml) and acidified slowly to pH 4 using dilute HCl (1 M) with stirring. The precipitate was obtained by vacuumfiltration, washed with water and dried, and was recrystallized from hot aqueous ethanol (water-ethanol, 1:4). In the case of N-hydroxycinnamoylanthranilate derivatives, water was added to the resulting solution to aid crystallization.α-Methylcinnamic acid - To a mixture of benzaldehyde (5 ml, 0.0492 mol) and propionic anhydride61 (10 ml, 0.0780 mol), anhydrous sodium acetate (2.5 g) was added and heated under reflux for 4 h. Once the mixture has cooled down to rt, cold water (50 ml) was added and alkalized with saturated aqueous sodium carbonate (85 ml). The resulting solid suspension was heated up to dissolve completely, and the unreacted benzaldehyde was extracted in DCM (2 × 25 ml) and the aqueous layer was acidified with concentrated HCl (12 M) with cooling. The pale yellowish white crystals (yield 2.098 g, 26 %) of α-methylcinnamic acid was obtained by vacuum filtration, washed with little cold water (~10 ml) and dried.
References:
Chandrabalan, Arundhasa;McPhillie, Martin J.;Morice, Alyn H.;Boa, Andrew N.;Sadofsky, Laura R. [European Journal of Medicinal Chemistry,2019,vol. 170,p. 141 - 156] Location in patent:supporting information
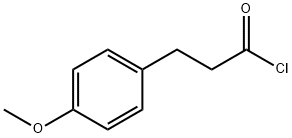
15893-42-2
74 suppliers
$20.00/250mg
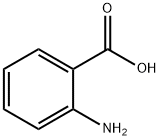
118-92-3
0 suppliers
$23.00/250g
![1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/StructureFile/ChemBookStructure8/GIF/CB1119552.gif)
692281-53-1
10 suppliers
inquiry
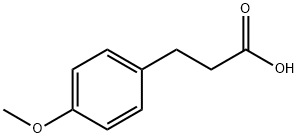
1929-29-9
241 suppliers
$8.00/5g
![1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/StructureFile/ChemBookStructure8/GIF/CB1119552.gif)
692281-53-1
10 suppliers
inquiry

15893-42-2
74 suppliers
$20.00/250mg
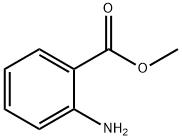
134-20-3
618 suppliers
$16.00/25mL
![1-ISOPROPYL-6-METHYL-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/StructureFile/ChemBookStructure8/GIF/CB1119552.gif)
692281-53-1
10 suppliers
inquiry