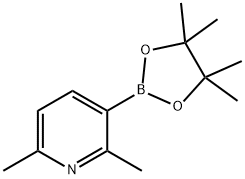
3-Hydroxy-2,3-diMethylbutan-2-yl hydrogen (2,6-diMethylpyridin-3-yl)boronate synthesis
- Product Name:3-Hydroxy-2,3-diMethylbutan-2-yl hydrogen (2,6-diMethylpyridin-3-yl)boronate
- CAS Number:693774-10-6
- Molecular formula:C13H20BNO2
- Molecular Weight:233.11
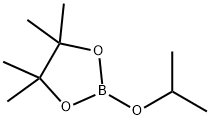
61676-62-8
312 suppliers
$10.00/5g

693774-10-6
69 suppliers
$50.00/25mg
Yield: 98.4%
Reaction Conditions:
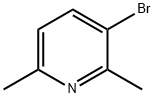
Stage #1:3-bromo-2,6-dimethylpyridine with n-butyllithium in diethyl ether;hexane at -78; for 0.75 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in diethyl ether;hexane at -78; for 3 h;
Steps:
2A
Example 2A 2,6-Dimethyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-pyridine A solution of 3-bromo-2,6-dimethylpyridine (5.10 g, 27.4 mmol) in anhydrous ether (160 ml) cooled to -78° C. under a nitrogen atmosphere was treated dropwise with n-butyl lithium (4.1 ml, 10 M in hexane) and stirred at -78° C. for 45 minutes. 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (10.2 g, 54.8 mmol) in 20 ml of ether was added dropwise at -78° C. and stirred at -78° C. for 3 hours. The mixture was quenched with 10 ml of isopropanol, and allowed to warm to room temperature. 150 ml of saturated aqueous NaCl solution was added. The aqueous phase was separated and extracted with dichloromethane (100 ml*6). The combined organic phases were dried and concentrated to provide the title compound as light brown oil (6.29 g, 98.4%). 1H NMR (300 MHz, CDCl3) δ ppm 7.92 (d, J=7.46 Hz, 1H) 6.96 (d, J=7.80 Hz, 1H) 2.73 (s, 3H) 2.53 (s, 3H) 1.34 (s, 12H). MS: (M+H)+=234.
References:
Cowart, Marlon D.;Sun, Minghua;Zhao, Chen;Zheng, Guo Zhu US2007/66588, 2007, A1 Location in patent:Page/Page column 29-30
3430-31-7
217 suppliers
$8.00/1g

73183-34-3
563 suppliers
$6.00/5g

693774-10-6
69 suppliers
$50.00/25mg