
Methyl 2-(4-((3-chloro-5-(trifluoromethyl)-2-pyridinyl)oxy)phenoxy)propanoate synthesis
- Product Name:Methyl 2-(4-((3-chloro-5-(trifluoromethyl)-2-pyridinyl)oxy)phenoxy)propanoate
- CAS Number:69806-40-2
- Molecular formula:C16H13ClF3NO4
- Molecular Weight:375.73

75-09-2
1209 suppliers
$10.00/25 mL

60075-04-9
22 suppliers
inquiry
497-19-8
1390 suppliers
$13.00/300G
72537-17-8
194 suppliers
$11.00/1g

69806-40-2
104 suppliers
$51.29/25 mg
Yield:-
Reaction Conditions:
with tetrabutylammomium bromide in water
Steps:
1 Preparation of Methyl 2-(4-((3-Chloro-5-trifluoromethyl-2-pyridinyl)oxy)-phenoxy)propionate STR4
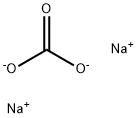
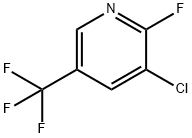
Example 1 Preparation of Methyl 2-(4-((3-Chloro-5-trifluoromethyl-2-pyridinyl)oxy)-phenoxy)propionate STR4 A 500 milliliter (mL) round-bottom flask was equipped with a mechanical paddle stirrer, an infrared heater connected to a thermocouple temperature controller and a drying tube. Methyl 2-(4-hydroxyphenoxy)propionate (103 grams (g): 0.5 moles) was introduced into the flask and heating was initiated to melt the solid. Stirring was begun and powdered anhydrous Na2 CO3 (74.2 g: 0.70 moles) was added. After 5 minutes (min), the temperature was about 80° C. and the evolution of gas was proceeding. Tetra-n-butylammonium bromide (4.8 g: 0.015 moles) was added, followed by 100 g (0.5 moles) of 2-fluoro-3-chloro-5-trifluoromethylpyridine. After 70 min at 80° C, the temperature was raised to 90° C. for an additional 230 min. The reaction mixture was allowed to cool and 750 mL of water and 500 mL of CH2 Cl2 were added. After mixing, the layers were separated. The aqueous layer was extracted with 50 mL of CH2 Cl2 and the combined organic layers were washed with 50 mL of water. The solvent was evaporated under reduced pressure to give 200.4 g of viscous oil. After 3 days, the oil began to crystallize. The product was recrystallized from methanol and dried to give 148.3 g of white solid, mp 55.5°-56.5° C.
References:
DowElanco US5049675, 1991, A