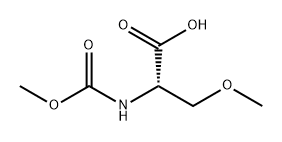
L-Serine, N-[(1,1-diMethylethoxy)carbonyl]-O-Methyl-, coMpd. with N-cyclohexylcyclohexanaMine (1:1) synthesis
- Product Name:L-Serine, N-[(1,1-diMethylethoxy)carbonyl]-O-Methyl-, coMpd. with N-cyclohexylcyclohexanaMine (1:1)
- CAS Number:69912-63-6
- Molecular formula:C21H40N2O5
- Molecular Weight:400.5527
Yield:-
Reaction Conditions:
Stage #1: boc-β-methoxyalanine dicyclohexylaminewith hydrogenchloride in 1,4-dioxane at 0 - 20;
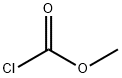
Stage #2: methyl chloroformatewith sodium carbonate;sodium hydroxide at 0 - 20;
Stage #3: with hydrogenchloride in water; pH=1;
Steps:
11
To Boc-O-methyl-L-serine dicyclohexylammonium salt 11a1 (Chem-lmpex) (4.5 g, 1 1.2 mmoles) in 1 ,4-dioxane at 0°C is added an 8M HCI solution in 1 ,4-dioxane and the mixture is stirred for 2 hours at RT. The reaction mixture is concentrated and co- distilled with 1 ,4-dioxane until dryness. The salt is dissolved in 1 N NaOH (22.5 ml_, 22.5 mmoles) and Na2C03 (0.6 g, 5.6 mmoles) is added at 0°C followed by dropwise addition of methyl chloroformate (0.87 ml_, 1 1.2 mmoles). The reaction mixture is stirred at RT for 3 hours. The solution is washed with ether and acidified (pH=1) with 1 N HCI. The aqueous layer is extracted with DCM and the organic layer is washed with brine, dried over Na2S04 and concentrated to afford 11a2.
References:
WO2011/91532,2011,A1 Location in patent:Page/Page column 26
![L-Serine, N-[(1,1-diMethylethoxy)carbonyl]-O-Methyl-, coMpd. with N-cyclohexylcyclohexanaMine (1:1)](/CAS/GIF/69912-63-6.gif)
69912-63-6
41 suppliers
inquiry

51293-47-1
199 suppliers
$8.00/250mg