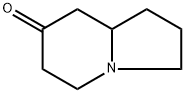
7(1H)-Indolizinone, hexahydro-, (-)- synthesis
- Product Name:7(1H)-Indolizinone, hexahydro-, (-)-
- CAS Number:275359-69-8
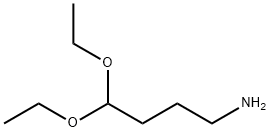
- Molecular formula:C8H13NO
- Molecular Weight:139.2
443797-19-1
1 suppliers
inquiry

275359-69-8
1 suppliers
inquiry
Yield:275359-69-8 93%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;water at 70; for 4 h;
Steps:
30.3 3) (+/-)-1,2,3,5,6,7,8,8a-Octahydroindolizin-7-one
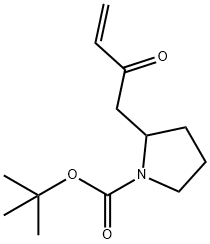
In 2 ml of tetrahydrofuran was dissolved 140 mg (0.59 mmol) of (+-)-1-(t-butoxycarbonyl)-2-(2-oxo-3-butenyl)pyrrolidine obtained in 2), and to this solution was added 1.76 ml (1.76 mmol) of 1N aqueous hydrochloric acid solution. The mixture was stirred at 70°C for 4 hours. Saturated aqueous sodium hydrogencarbonate solution was added to this reaction mixture and the mixture was extracted with dichloromethane. The organic layer was washed with water, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give 76 mg of the title compound as a slightly brown oil (yield: 93%). 1H-NMR Spectrum (500 MHz, CDCl3) δ ppm: 3.36-3.30 (1H, m), 3.19-3.13 (1H, m), 2.69-2.58 (1H, m), 2.55-2.50 (1H, m), 2.38-2.19 (5H, m), 2.02-1.93 (2H, m), 1.87-1.80 (1H, m), 1.59-1.50 (1H, m).
References:
EP1541571,2005,A1 Location in patent:Page/Page column 96
![1-Pyrrolidinecarboxylic acid, 2-[4-[(4-methylphenyl)sulfonyl]-2-oxobutyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/287730-08-9.gif)
287730-08-9
0 suppliers
inquiry

275359-69-8
1 suppliers
inquiry
![2-Butanone, 4-[(4,4-diethoxybutyl)amino]-](/CAS/20210305/GIF/87265-36-9.gif)
87265-36-9
0 suppliers
inquiry

275359-69-8
1 suppliers
inquiry