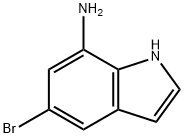
7-AMINO-5-BROMOINDOLE synthesis
- Product Name:7-AMINO-5-BROMOINDOLE
- CAS Number:374537-99-2
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06

374537-99-2
54 suppliers
$45.00/10mg
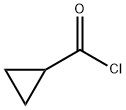
4023-34-1
356 suppliers
$12.00/5g
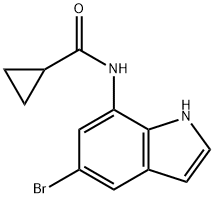
1610801-24-5
1 suppliers
inquiry
Yield:1610801-24-5 76%
Reaction Conditions:
with triethylamine in dichloromethane; for 2 h;Cooling with ice;
Steps:
23 N-(5-(4-Amino-5-oxo-7,8-dihydropyrimido[5,4-f][1,4]oxazepin-6(5H)-yl)-1-(2-methoxyphenyl)-1H-indol-7-yl)cyclopropanecarboxamide
N-(5-Bromo-1H-indol-7-yl)cyclopropanecarboxamide (23A)
A solution of cyclopropanecarbonyl chloride (0.54 g, 5.21 mmol) in DCM (10 mL) was added dropwise to an ice-cold solution of 5-bromo-1H-indol-7-amine (1.0 g, 4.74 mmol) and triethylamine (0.991 mL, 7.11 mmol) in DCM (20 mL), and the mixture was stirred for 2 h.
The reaction mixture was concentrated in vacuo and partitioned between ethyl acetate and water.
Separated organic layer was dried over sodium sulphate and filtered, and the filtrate was concentrated in vacuo.
The residue was purified by flash chromatography using 20% ethyl acetate in hexanes to afford the title compound (1.1 g, 76%) as a red solid. 1H NMR (300 MHz, DMSO-d6): δ 11.0 (s, 1H), 10.08 (s, 1H), 7.72 (s, 1H), 7.48 (s, 1H), 7.41 (t, J=3.0 Hz, 1H), 6.43 (t, J=2.4 Hz, 1H), 1.90-1.86 (m, 1H), 0.88-0.82 (m, 4H). ESI-MS m/z=279 (M+H)+.
References:
US2014/148437,2014,A1 Location in patent:Page/Page column 0258-0260

374537-99-2
54 suppliers
$45.00/10mg
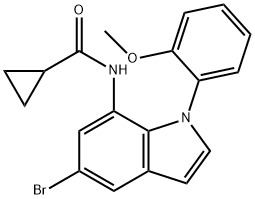
1610801-26-7
1 suppliers
inquiry