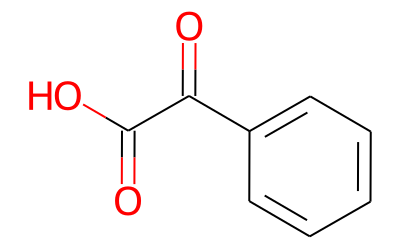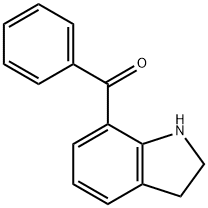
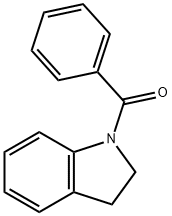
7-Benzoylindoline synthesis
- Product Name:7-Benzoylindoline
- CAS Number:33244-57-4
- Molecular formula:C15H13NO
- Molecular Weight:223.27
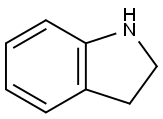
496-15-1
426 suppliers
$8.00/10g
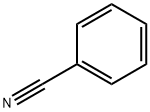
100-47-0
359 suppliers
$14.14/25gm:

33244-57-4
20 suppliers
inquiry
Yield:33244-57-4 100%
Reaction Conditions:
Stage #1: 1-indoline;benzonitrilewith aluminum (III) chloride;water;boron trichloride in dichloromethane;toluene at 110;
Stage #2: with hydrogenchloride;water in dichloromethane;toluene at 80; for 1 h;
Steps:
9.1 Example 9-1 Preparation of indolin-7-yl (phenyl) methanone
A 250 mL 2-neck round bottom flask was connected to a water-cooled condenserIndoline 5.7 g (48 mmol) was added and purified tolueneAnd then dissolved in 48 mL of water. After cooling the solution to 0 ° C, 53 mL (1 M solution in methylene chloride, 53Mmol, 1 eq.) Was added and stirred. Benzonitrile (10 mL, 96 mmol, 2 eq) and AlCl3 (6.4 g, 48 mmol, 1 eq) were added and refluxed overnight at 110 & lt; 0 & gt; C. After the reaction was completed, the reaction mixture was vacuum-dried at 110 ° C to remove the solvent, cooled to room temperature,N HCl (100 mL) was added, and the mixture was stirred for 1 hour at 80 ° C. After cooling to room temperature, the reaction mixture was dissolved in methylene chloride, and the organic layer was collected. Transfer the collected organic layer to a separatory funnelAfter the addition of 1N KOH, the pH of the water layer was adjusted to 9 ~ 10. The organic layer collected after extraction was washed with brineWashed, dried over Na2SO4, filtered and concentrated to give a yellow solid crude product. A small amount of methylene chlorideThe residue was recrystallized with excess methanol and filtered to obtain 11 g (quantitative yield) of a yellow solid..
References:
KR101648137,2016,B1 Location in patent:Paragraph 0206-0208

496-15-1
426 suppliers
$8.00/10g

33244-57-4
20 suppliers
inquiry
61589-14-8
29 suppliers
inquiry

33244-57-4
20 suppliers
inquiry

98-88-4
575 suppliers
$10.00/5g

33244-57-4
20 suppliers
inquiry