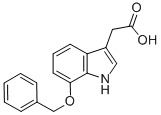
(7-BENZYLOXY-1H-INDOL-3-YL)-ACETIC ACID synthesis
- Product Name:(7-BENZYLOXY-1H-INDOL-3-YL)-ACETIC ACID
- CAS Number:99102-25-7
- Molecular formula:C17H15NO3
- Molecular Weight:281.31

99102-24-6
2 suppliers
inquiry

99102-25-7
17 suppliers
inquiry
Yield:99102-25-7 86%
Reaction Conditions:
Stage #1: (7-benzyloxy-indol-3-yl)-acetonitrilewith sodium hydroxide in methanol;water; for 5 h;Heating / reflux;
Stage #2: with hydrogenchloride in methanol;water;
Steps:
20 (7-(Benzyloxy)-1H-indol-3-yl)acetic acid
Under nitrogen atmosphere, to a suspension of (7-(benzyloxy)-1H-indol-3-yl)acetonitrile (13.2 g, 50.3 mmol) in methanol (400 mL) is added 10N aqueous sodium hydroxide solution (130 mL), and the mixture is refluxed for 5 hours. The reaction solution is cooled to room temperature, and methanol alone is distilled off. The pH value of the resultant is adjusted to pH 1 with conc. hydrochloric acid while the mixture is cooled with ice. The precipitates are collected by filtration, and dissolved in chloroform, and dried over anhydrous sodium sulfate. The solvent is evaporated to give (7-(benzyloxy)-1H-indol-3-yl)acetic acid (12.2 g, 43.4 mmol, yield: 86 %). 1H-NMR (DMSO-d6) δ: 12.09 (1H, brs), 11.04 (1H, s), 7.55-7.57 (2H, m), 7.39-7.42 (2H, m), 7.31-7.35 (1H, m), 7.14 (1H, d, J=2.4Hz), 7.09 (1H, d, J=7.9Hz), 6.88 (1H, dd, J=7.9, 7.6Hz), 6.73 (1H, d, J=7.6Hz), 5.26 (2H, s), 3.61 (2H, s).
References:
EP1514869,2005,A1 Location in patent:Page/Page column 31
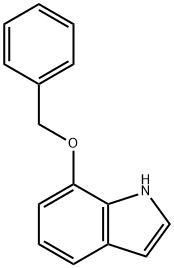
20289-27-4
229 suppliers
$10.00/250mg

99102-25-7
17 suppliers
inquiry