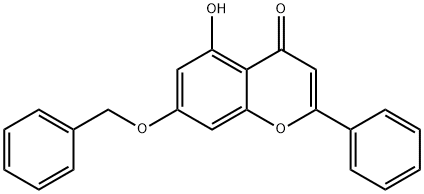
7-(benzyloxy)-5-hydroxy-2-phenyl-4H-chromen-4-one synthesis
- Product Name:7-(benzyloxy)-5-hydroxy-2-phenyl-4H-chromen-4-one
- CAS Number:110506-85-9
- Molecular formula:C22H16O4
- Molecular Weight:344.36
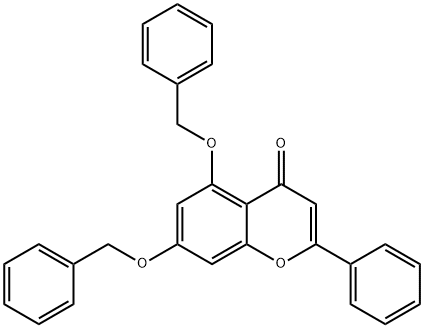
137790-18-2
2 suppliers
inquiry

110506-85-9
17 suppliers
inquiry
Yield:110506-85-9 98%
Reaction Conditions:
with titanium tetrachloride in dichloromethane at 0 - 20; for 2 h;
Steps:
4.3.3. 5-Benzoyloxy-7-benzyloxyflavone (1j)
A solution of TiCl4 (1 M in CH2Cl2, 0.31 mL, 0.31 mmol) was added dropwise to a solution of 5,7-dibenzyloxyflavone (1k)5 (0.220 g, 0.476 mmol) in anhydrous CH2Cl2 (5 mL) at 0 °C and the reaction mixture was allowed to warm to room temperature over 2 h. The reaction mixture was carefully added to a stirred solution of saturated aqueous NaHCO3 (30 mL) and extracted with CH2Cl2 (3×50 mL). The combined organic layers were dried (Na2SO4), filtered and the volatiles were removed in vacuo to afford crude 7-benzyloxy-5-hydroxyflavone (0.170 g, 98%) as an orange solid, which was used without further purification. Benzoyl chloride (0.5 mL) was then added to a solution of this flavone (0.17 g, 0.51 mmol) in anhydrous pyridine (3 mL) at 0 °C and the reaction mixture was stirred at 60 °C for 5 h then at room temperature for 48 h. The reaction mixture was quenched with methanol and the volatiles were removed in vacuo. The residue was diluted with CH2Cl2 (20 mL) and the organic phase was washed with dil HCl (1 M, 20 mL), brine (20 mL), dried (MgSO4), filtered and volatiles were removed in vacuo. The crude product was purified by column chromatography on silica gel (20 g, CH2Cl2 elution) to afford 1j (0.180 g, 79%) as a white solid; mp 137-138 °C; 1H NMR (500 MHz, CDCl3) δH 5.20 (s, 2H, PhCH2O), 6.60 (s, 1H, H-3), 6.85 (d, J=2.5 Hz, 1H, H-6), 7.02 (d, J=2.5 Hz, 1H, H-8), 7.43 (m, 5H, H-2, 6), 7.52 (m, 3H, H-3', 4', 5'), 7.52 (dd, J=8.5, 8.5 Hz, 2H, H-3, 5), 7.64 (dd, J=8.5, 8.5 Hz, 1H, H-4), 7.85 (dd, J=1.0, 7.5 Hz, 2H, H-2', 6'), 8.26 (dd, J=1.5, 8.5 Hz, 2H, H-2, 6); 13C NMR (125 MHz, CDCl3) δC 70.9 (t, PhCH2O), 100.2 (d, C-8), 108.6 (d, C-3), 109.2 (d, C-6), 111.7 (s, C-4a), 126.2 (2×d, C-2', 6'), 127.7 (2×d, C-2, 6), 128.6 (2×d, C-3, 5), 128.6 (d, C-4), 128.9 (2×d, C-3, 5), 129.1 (3×d, C-3', 4', 5'), 129.9 (s, C-1), 130.5 (2×d, C-2, 6), 131.5 (s, C-1'), 133.4 (d, C-4), 135.5 (s, C-1), 150.9 (s, C-5), 158.9 (s, C-8a), 162.1 (s, C-2), 162.7 (s, C-7), 165.4 (s, PhCOO), 176.5 (s, C-4); IR (thin film) νmax 3062, 1740, 1645, 1493, 1450, 1375, 1351, 1262, 1159, 1093, 1058, 1028, 696 cm-1; HRMS (+ve ESI) m/z 449.1365 [M+H]+ (calcd for C29H21O5 [M+H]+ 449.1384).
References:
Compton, Benjamin J.;Larsen, Lesley;Weavers, Rex T. [Tetrahedron,2011,vol. 67,# 4,p. 718 - 726] Location in patent:experimental part
![Ethanone, 1-[2-(benzoyloxy)-4,6-bis(phenylmethoxy)phenyl]-](/CAS/20210305/GIF/137790-51-3.gif)
137790-51-3
0 suppliers
inquiry

110506-85-9
17 suppliers
inquiry
480-40-0
556 suppliers
$11.00/5g

100-44-7
661 suppliers
$13.50/250G

110506-85-9
17 suppliers
inquiry

480-40-0
556 suppliers
$11.00/5g

100-44-7
661 suppliers
$13.50/250G

137790-18-2
2 suppliers
inquiry

110506-85-9
17 suppliers
inquiry
