
7-BENZYLOXYTRYPTAMINE synthesis
- Product Name:7-BENZYLOXYTRYPTAMINE
- CAS Number:31677-75-5
- Molecular formula:C17H18N2O
- Molecular Weight:266.34
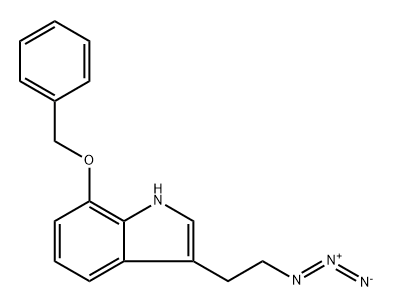
639083-75-3
0 suppliers
inquiry

31677-75-5
25 suppliers
$50.00/50mg
Yield:31677-75-5 95%
Reaction Conditions:
with triphenylphosphine in pyridine;water at 20; for 18 h;
Steps:
79 2-(7-(Benzyloxy)-1H-indol-3-yl)ethylamine
To a solution of 3-(2-azidethyl)-7-(benzyloxy)-1H-indole (5.10 g, 17.4 mmol) in pyridine (100 mL)-water (100 mL) is added triphenylphosphine (5.02 g, 19.1 mmol) under nitrogen atmosphere, and the mixture is stirred at room temperature for 18 hours. The reaction solution is poured into water, and the mixture is extracted with chloroform. The organic layer is washed with a saturated brine, and dried over anhydrous magnesium sulfate. The solvent is evaporated under reduced pressure, and the obtained crude product is purified by silica gel column chromatography (a saturated ammonia solution in chloroform → a saturated ammonia solution in chloroform-methanol = 50:1 → 10: 1) to give the title compound (4.38 g, yield: 95 %). 1H-NMR (CDCl3) δ: 2.89 (2H, t, J=6.8Hz), 3.02 (2H, t, J=6.8Hz), 5.21 (2H, s), 6.73 (1H, d, J=7.7Hz), 7.01 (1H, d, J=2.1Hz), 7.02 (1H, dd, J=8.0, 7.7Hz), 7.24 (1H, d, J=8.0Hz), 7.34-7.43 (3H, m), 7.47-7.49 (2H, m), 8.29 (1H, brs).
References:
EP1514869,2005,A1 Location in patent:Page/Page column 69
![1H-Indole, 3-[(1E)-2-nitroethenyl]-7-(phenylmethoxy)-](/CAS/20210305/GIF/1196967-49-3.gif)
1196967-49-3
0 suppliers
inquiry

31677-75-5
25 suppliers
$50.00/50mg
858232-85-6
0 suppliers
inquiry

31677-75-5
25 suppliers
$50.00/50mg
20289-27-4
229 suppliers
$10.00/250mg

31677-75-5
25 suppliers
$50.00/50mg
94067-27-3
44 suppliers
$74.50/50mg

31677-75-5
25 suppliers
$50.00/50mg