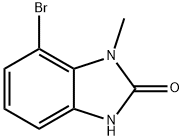
7-bromo-1-methyl-1,3-dihydro-2H-benzimidazol-2-one synthesis
- Product Name:7-bromo-1-methyl-1,3-dihydro-2H-benzimidazol-2-one
- CAS Number:913297-44-6
- Molecular formula:C8H7BrN2O
- Molecular Weight:227.06
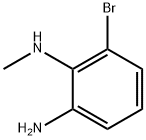
1150102-47-8
52 suppliers
$45.00/10mg

913297-44-6
72 suppliers
inquiry
Yield: 88%
Reaction Conditions:
with 1,1'-carbonyldiimidazole in acetonitrile at 85; for 12 h;Inert atmosphere;
Steps:
3 Step 3-4-Bromo-3-methyl-1H-benzimidazol-2-one
To a mixture of 3-bromo-N2-methyl-benzene-1,2-diamine (20.0 g, 99.4 mmol) in ACN (300 mL) was added CDI (32.2 g, 198 mmol). The reaction mixture was stirred at 85° C. for 12 hours under N2 atmosphere. On completion, the reaction mixture was concentrated in vacuo. The reaction mixture was diluted with water (200 mL), where a solid precipitate was formed, which was filtered off. The solid was washed with water (1 L) and dried in vacuo to give the title compound (20.0 g, 88% yield) as white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.17 (s, 1H), 7.14 (dd, J=1.2, 8.0 Hz, 1H), 7.00-6.95 (m, 1H), 6.93-6.87 (m, 1H), 3.55 (s, 3H).
References:
Kymera Therapeutics, Inc.;Mainolfi, Nello;Ji, Nan;Kluge, Arthur F.;Weiss, Matthew M.;Zhang, Yi US2019/192668, 2019, A1 Location in patent:Paragraph 2881; 2884

1150102-47-8
52 suppliers
$45.00/10mg
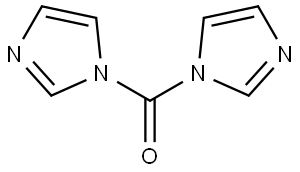
530-62-1
925 suppliers
$6.00/25g

913297-44-6
72 suppliers
inquiry

851886-92-5
3 suppliers
inquiry

913297-44-6
72 suppliers
inquiry

1004618-77-2
46 suppliers
inquiry

913297-44-6
72 suppliers
inquiry
58534-94-4
184 suppliers
$10.00/1g

913297-44-6
72 suppliers
inquiry