
7-bromo-2-chloro-6-methoxybenzo[d]thiazole synthesis
- Product Name:7-bromo-2-chloro-6-methoxybenzo[d]thiazole
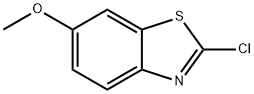
- CAS Number:911056-05-8
- Molecular formula:C8H5BrClNOS
- Molecular Weight:278.55
2605-14-3
163 suppliers
$39.00/1g
![7-bromo-2-chloro-6-methoxybenzo[d]thiazole](/CAS/20180629/GIF/911056-05-8.gif)
911056-05-8
28 suppliers
inquiry
Yield:911056-05-8 60%
Reaction Conditions:
with N-Bromosuccinimide in 1-methyl-pyrrolidin-2-one at 20 - 75; for 24 h;
Steps:
195.1 Step 1.
Step 1. Synthesis of 7-bromo-2-chloro-6-methoxybenzo[d]thiazole To the solution of 2-chloro-6-methoxy-benzothiazole (200 mg, 1.0 mmol, 1.0 eq) in 5 mL of NMP was added N-bromosuccinimide (213 mg, 1.20 mmol, 1.2 eq) at room temperature. The reaction mixture was stirred at 75° C. for >24 hours with subsequent addition of NBS in small batches for reaction progress, thereafter the mixture was diluted with water (ca. 100 mL) and aqueous layer extracted with ethyl acetate (ca. 150 mL*3). Combined organic layers were dried over sodium sulfate, filtered and condensed under reduced pressure. Purification on ISCO using gradient 0%-50% ethyl acetate-hexane gave 336 mg of product as off white fluffy powder in 60% yields structure of which was confirmed by H+NMR. LC/MS (m/z) [279.9] (MH+)
References:
US2008/45528,2008,A1 Location in patent:Page/Page column 95-96