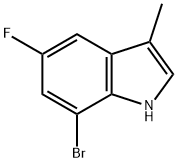
7-Bromo-5-fluoro-3-methyl-1H-indole synthesis
- Product Name:7-Bromo-5-fluoro-3-methyl-1H-indole
- CAS Number:883001-24-9
- Molecular formula:C9H7BrFN
- Molecular Weight:228.06
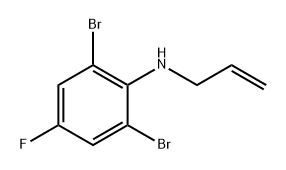
946197-48-4
0 suppliers
inquiry

883001-24-9
28 suppliers
$240.00/100mg
Yield:883001-24-9 77%
Reaction Conditions:
with triethylamine;palladium diacetate;tris-(o-tolyl)phosphine in acetonitrile; for 2 - 3 h;Product distribution / selectivity;Heating / reflux;
Steps:
1.2; 2; 3
Step 2. 7-Bromo-5-fluoro-3-methylindole; To a solution of N-Allyl-2,6-dibromo-4-fluoroaniline (20 g, 65 mmol) in 100 mL acetonitrile was added palladium(II) acetate (150 mg, 0.7 mmol), tri-O-tolylphosphine (600 mg, 2 mmol) and triethylamine (26.3 g, 260 mmol), and the resulting solution was heated at reflux for 2.5 h. The reaction was cooled to room temperature and filtered through a celite mat. The celite was rinsed with 25 mL acetonitrile, and the combined solutions were concentrated in vacuo to provide 22.5 g of crude product. The product was purified via silica gel column chromoatography to afford 11.3 g (77% yield) of the title compound: 1H NMR (400 MHz, CDCl3) δ 2.27 (d, 3H, J=1.2 Hz), 7.06 (br s, 1H), 7.14 (dd, 1H, J=8.8, 2.4 Hz), 7.18 (dd, 1H, J=8.8, 2.4 Hz), 8.01 (br, 1H).; Example 2; Preparation of methyl 3-(5-fluoro-3-methylindol-7-yl)acrylate via Heck Coupling without isolation of 7-haloindole intermediate; To a solution of N-allyl-2,6-dibromo-4-fluoroaniline (23.0 g, 74.4 mmol), prepared as in Step 1 of Example 1, in anhydrous acetonitrile (115 mL) in a 3-neck 250 mL flask fitted with a condenser, temperature probe, heating mantle, and nitrogen bubbler was added palladium(II) acetate (167 mg, 0.744 mmol), tri-O-tolylphosphine (906 mg, 3.0 mmol), and triethylamine (15.6 mL, 110 mmol). The dark solution was refluxed under nitrogen. After 2 h, TLC analysis indicated that the starting material was consumed. After two additional h the reaction mixture was cooled to 40° C., and the solution was charged with palladium(II) acetate (167 mg), tri-O-tolylphosphine (906 mg), triethylamine (15.6 mL), and methyl acrylate (13.4 mL, 149 mmol), and reflux was resumed. After cooling to room temperature the reaction mixture was diluted with MTBE (200 mL) and water (200 mL), and the mixture was stirred for 10 min. The dark upper organic layer was separated and washed with water (3×100 mL), brine (100 mL), and dried over sodium sulfate. After filtration the solvent was removed to obtain a tan solid. The material was dried at 50° C. for 2 h, providing 19.3 g (111%) of crude product. The crude material was suspended in a mixture of MTBE (60 mL) and hexanes (100 mL), and the mixture was refluxed for 2 h. After cooling to room temperature a gray-colored solid was collected by filtration, washed well with hexane (200 mL), and dried under vacuum at 45-50° C. for 60 hrs, providing 7.2 g of the desired product: 1H NMR (400 MHz, CDCl3) δ 2.29 (d, 3H, J=1.2 Hz), 3.84 (s, 3H), 6.49 (d, 1H, J=16 Hz), 7.07 (br s, 1H), 7.15 (dd, 1H, J=10, 2.4 Hz), 7.27 (dd, 1H, J=9.2, 2.4 Hz), 7.95 (d, 1H, J=16 Hz), 8.35 (br s, 1H).; Example 3; Preparation of 3-(5-fluoro-3-methylindol-7-yl)acrylic acid via Heck coupling without isolation of 7-haloindole intermediate; To a solution of N-allyl-2,6-dibromo-4-fluoroaniline (2.09 g, 6.76 mmol), prepared as in Step 1 of Example 1, in anhydrous acetonitrile (15 mL) was added palladium(II) acetate (31.4 mg, 0.137 mmol), tri-O-tolylphosphine (120 mg, 0.383 mmol), and triethylamine (3.8 mL, 27.3 mmol). The reaction was heated at reflux for 3 h, at which point TLC indicated consumption of starting material. The reaction was cooled to room temperature, then acrylic acid (0.56 mL, 8.08 mmol) was added via syringe and refluxing was resumed. After 3.5 h at reflux, TLC indicated reaction completion. The solution was cooled to room temperature, diluted with 21 mL water, then approximately 10 mL of the solvent was evaporated in vacuo. The solution was diluted with additional water and washed with MTBE (2×10 mL). The separated aqueous solution was acidified to pH 2-3 with 1 M HCl, which induced precipitation of the product as a yellow solid. The product was collected via suction filtration, washed with water, then vacuum dried overnight at 47° C., providing the title compound as a bright yellow solid (1.33 g, 90% yield): 1H NMR (400 MHz, DMSO-d6) δ 2.23 (d, 3H, J=0.8 Hz), 6.67 (d, 1H, J=16 Hz), 7.24 (br s, 1H), 7.34 (dd, 1H, J=9.2, 2.4 Hz), 7.41 (dd, 1H, J=10.4, 2.4 Hz), 8.06 (dd, 1H, J=16, 1.2 Hz), 11.35 (s, 1H).
References:
US2007/270594,2007,A1 Location in patent:Page/Page column 8-9
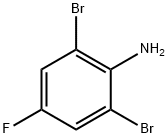
344-18-3
239 suppliers
$11.00/10g

883001-24-9
28 suppliers
$240.00/100mg