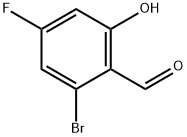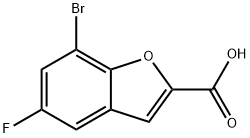
7-BROMO-5-FLUOROBENZOFURAN-2-CARBOXYLIC ACID synthesis
- Product Name:7-BROMO-5-FLUOROBENZOFURAN-2-CARBOXYLIC ACID
- CAS Number:550998-61-3
- Molecular formula:C9H4BrFO3
- Molecular Weight:259.03
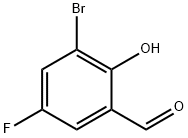
178546-34-4
50 suppliers
$22.00/100mg

96-34-4
338 suppliers
$10.00/25g

550998-61-3
10 suppliers
inquiry
Yield:550998-61-3 63%
Reaction Conditions:
Stage #1: 3-bromo-5-fluoro-2-hydroxybenzaldehyde;methyl chloroacetatewith potassium carbonate;tetra-(n-butyl)ammonium iodide at 130; for 4 h;
Stage #2: with potassium hydroxide in tetrahydrofuran;water at 0 - 20; for 18 h;
Stage #3: with hydrogenchloride in water;
Steps:
50b
Methyl chloroacetate (7.6 mL, 86.8 mmol) was added to a mixture of 3-bromo-5-fluoro-2-hydroxy-benzaldehyde (9.5 g, 43.4 mmol), tetra-N-butyl-ammonium iodide (1.6 g, 4.34 mmol) and anhydrous potassium carbonate (24 g, 174 mmol). The reaction mixture was heated at 130° C. for 4 h, cooled to 0° C., diluted with tetrahydrofuran (100 mL) and then potassium hydroxide (17 g, 303 mmol) in water (100 mL). The mixture was stirred at rt for 18 h and then, concentrated under reduced pressure. The residue was acidified with HCl and extracted with ethyl acetate. The combined organic extracts were dried (Na2SO4), filtered and concentrated under reduced pressure. The crude compound was purified by flash chromatography (SiO2; toluene/EtOAc, 40/1) to give 7.0 g (63%) of the product.1H NMR (400 MHz, methyl alcohol-d4) δ ppm 7.66 (s, 1H), 7.47-7.54 (m, 2H).
References:
US2010/331341,2010,A1 Location in patent:Page/Page column 32
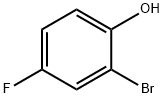
496-69-5
282 suppliers
$6.00/5g

550998-61-3
10 suppliers
inquiry