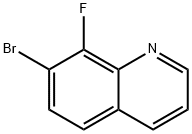
7-bromo-8-fluoroquinoline synthesis
- Product Name:7-bromo-8-fluoroquinoline
- CAS Number:1375107-95-1
- Molecular formula:C9H5BrFN
- Molecular Weight:226.05

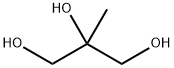
58534-95-5
177 suppliers
$8.00/1g
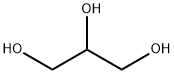
56-81-5
1651 suppliers
$5.00/25g

1375107-95-1
17 suppliers
inquiry
Yield:1375107-95-1 90%
Reaction Conditions:
with sodium 3-nitrobenzenesulfonate in water at 120;Inert atmosphere;
Steps:
Intermediate 30: 7-bromo-8-fluoroquinoline
H2SO4 (4 mL) was added to 3-bromo-2-fluoroaniline (100 mg, 421 μmol), sodium 3- nitrobenzene-1-sulfonate (118 mg, 526 μmol), propane-1,2,3-triol (2.5 mL) and H2O (2 mL) at rt. The reaction was stirred at 120°C for 6 hr under N2. The resulting mixture was poured into ice-water and adjusted to pH= 8 with sat NaOH (aq). The mixture was diluted with EA (100 mL) and washed with brine (50 mL *2). The organic layer was dried with Na2SO4 and concentrated under vacuum. The residue was purified by a silica gel column using DCM: MeOH = 25: 1. This resulted in the title compound (90 mg, yield: 90%) as a yellow solid. LC-MS: (ES, m/z): RT = 1.179min, LCMS: m/z = 226[M+1].
References:
WO2022/192431,2022,A1 Location in patent:Page/Page column 83