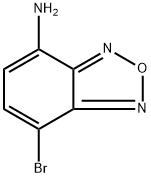
7-Bromobenzo[c][1,2,5]oxadiazol-4-amine synthesis
- Product Name:7-Bromobenzo[c][1,2,5]oxadiazol-4-amine
- CAS Number:406224-62-2
- Molecular formula:C6H4BrN3O
- Molecular Weight:214.02
![4-BroMo-7-nitrobenzo[c][1,2,5]oxadiazole](/CAS/20150408/GIF/35128-56-4.gif)
35128-56-4
23 suppliers
inquiry
![7-Bromobenzo[c][1,2,5]oxadiazol-4-amine](/CAS/20211123/GIF/406224-62-2.gif)
406224-62-2
1 suppliers
inquiry
Yield:406224-62-2 470 mg (95%)
Reaction Conditions:
in acetic acid;ethyl acetate;
Steps:
422.B B.
B. 4-Bromo-7-aminobenzofurazan (422B) A solution of compound 422A (563 mg, 2.31 mmol) in AcOH (5 mL) was heated to 70° C. and Fe0 powder (258 mg, 4.62 mmol) was added in one portion. The resulting dark reaction mixture was stirred for 15 min, cooled to rt and concentrated under reduced pressure. The residue was taken up in EtOAc and the resulting solution was washed with sat. Na2CO3 solution. The organic layer was dried over Na2SO4, concentrated in vacuo and purified by flash chromatography on silica gel eluding with 10-60% EtOAc in hexanes to give 470 mg (95%) of compound 422B as a red solid.
References:
US2004/77605,2004,A1 US2005/119228,2005,A1