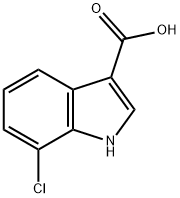
7-CHLORO-1H-INDOLE-3-CARBOXYLIC ACID synthesis
- Product Name:7-CHLORO-1H-INDOLE-3-CARBOXYLIC ACID
- CAS Number:86153-24-4
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6
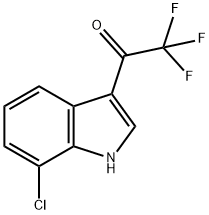
934185-80-5
3 suppliers
inquiry

86153-24-4
67 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
Stage #1: 7-chloroindolewith sodium hydroxide;water for 1 h;Heating / reflux;
Stage #2: with hydrogenchloride;water pH=1;
Steps:
1.A
EXAMPLE 1; 3-({5-[4-(Carbamoylmethyl)piperazin-1-yl]methyl}-[1,2,4]-thiadiazol-3-yl)-7-chloro-1-(tetrahydropyran-4-yl)methyl-1H-indole, bis-hydrochloride salt; Step A: 7-Chloro-1H-indole-3-carboxylic acidA solution of 7-chloroindole (7.1 g, 47.0 mmol) in dimethylformamide (60 ml) was cooled to 5° C. under nitrogen and trifluoroacetic anhydride (7.6 ml, 54.0 mmol) was added over 10 mins, maintaining the temperature below 10° C. The mixture was stirred at 5-10° C. for 2 h, then poured into water (600 ml). The resulting suspension was stirred for 15 mins and the 7-chloro-3-[(trifluoromethyl)carbonyl]-1H-indole precipitate was filtered off, washing with water to neutrality. The damp solid was suspended in 4 M aqueous sodium hydroxide (500 ml) and heated to reflux with stirring for 1 h. The mixture was cooled and washed with diethyl ether (2×100 ml). The aqueous phase was then acidified to pH 1 using 5 M hydrochloric acid and the resulting fine precipitate filtered off, washed with water to neutrality and dried to afford 7-chloro-1H-indole-3-carboxylic acid as a pink solid (7.5 g, 38.0 mmol).
References:
US2008/207598,2008,A1 Location in patent:Page/Page column 7-8
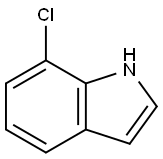
53924-05-3
306 suppliers
$14.14/250mgs:

86153-24-4
67 suppliers
$45.00/50mg