
7-CHLORO-5-METHOXY-6-AZAINDOLE synthesis
- Product Name:7-CHLORO-5-METHOXY-6-AZAINDOLE
- CAS Number:930790-40-2
- Molecular formula:C8H7ClN2O
- Molecular Weight:182.61
38533-61-8
298 suppliers
$8.00/5g
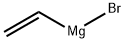
1826-67-1
208 suppliers
$60.89/100ml

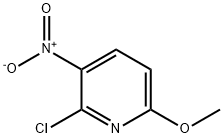
930790-40-2
26 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-chloro-6-methoxy-3-nitropyridine;vinyl magnesium bromide in tetrahydrofuran at -20; for 8 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;
Steps:
43.1
Step 1 2-Chloro-6-methoxy-3-nitropyridine (5 g, 0.027 mol) was dissolved in anhydrous tetrahydrofuran (200 mL) under nitrogen and the solution was cooled to -78° C. Excess of vinylmagnesium bromide (1.0 M in tetrahydrofuran, 100 mL, 100 mmol) was added and the reaction mixture was stirred at -20° C. for 8 hours and then the reaction mixture was quenched with 20% aqueous ammonium chloride (150 mL). The aqueous layer was extracted with ethyl acetate and the combined extracts were dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified via Biotage Horizon (FlasH 40 M, silica, gradient from 20% ethyl acetate/hexane to 60% ethyl acetate/hexane) to yield 7-chloro-5-methoxy-1H-pyrrolo[2,3-c]pyridine2 as a yellow solid. MS (ESI) m/z 183 ([M+H]+). 2 Zhang, Z.; et al., J. Org. Chem. 2002, 76, 2345-2347.
References:
US2007/72897,2007,A1 Location in patent:Page/Page column 57