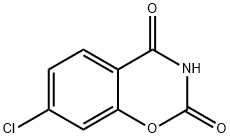
7-chloro-benzo[e][1,3]oxazine-2,4-dione synthesis
- Product Name:7-chloro-benzo[e][1,3]oxazine-2,4-dione
- CAS Number:31544-40-8
- Molecular formula:C8H4ClNO3
- Molecular Weight:197.58
Yield:31544-40-8 83%
Reaction Conditions:
with pyridine in water;acetonitrile;
Steps:
1.c 1c.
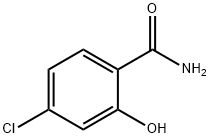
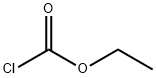
Acetonitrile (4700 mL) and 4-chloro-2-hydroxybenzamide (1782 g, 10.4 mol) were charged to the reaction flask and the stirring was started. Pyridine (1133 mL, 14.0 mol) was charged to the reactor. The resulting reaction slurry was cooled to less than 10° C. with an ice bath. Ethyl chloroformate (1091 mL, 1237 g, 11.4 mol) was placed in the addition funnel and charged slowly to the stirred reaction mixture such that the temperature of the reaction mixture did not exceed 15° C. during the addition. The temperature of the reaction mixture was held between 10 and 15° C. for 30 minutes after the ethyl chloroformate addition was complete. The ice bath was removed, and the reaction mixture was warmed to ambient temperature. The reaction mixture was then slowly heated to reflux and held at that temperature for 18 hours. Liquid chromatographic analysis of the reaction mixture indicated that the reaction was only 80% complete. Approximately half of the solvent was removed by atmospheric distillation. The reaction mixture was cooled first to ambient temperature and then to <10° C. with an ice bath. Additional pyridine (215 mL, 2.65 mol) was added to the reaction mixture. Ethyl chloroformate (235 g, 2.17 mol) was added slowly via an addition funnel to the cold reaction mixture. The reaction mixture was held between 10 and 15° C. for 30 minutes after the ethyl chloroformate addition was complete. The ice bath was removed, and the reaction mixture was warmed to ambient temperature. The reaction mixture was then slowly heated to reflux and held at that temperature for 18 hours, after which time liquid chromatographic analysis indicated that the reaction was complete. The reaction mixture was cooled first to ambient temperature and then to <10° C. with an ice bath. Water (1600 mL) was added slowly via an addition funnel and the resulting slurry held at <10° C. for 90 minutes. The solid product was collected by vacuum filtration through a large sintered glass funnel. The product filter cake was washed with deionized water and vacuum dried at 50° C. for 18 hours to give 1914 g of 7-chloro-2H-1,3-benzoxazine-2,4(3H)-dione as a tan solid. The yield was 83%.
References:
US2004/48777,2004,A1