
7-CHLOROPYRIDO[3,2-D]PYRIMIDIN-4(3H)-ONE synthesis
- Product Name:7-CHLOROPYRIDO[3,2-D]PYRIMIDIN-4(3H)-ONE
- CAS Number:897360-16-6
- Molecular formula:C7H4ClN3O
- Molecular Weight:181.58

27330-34-3
39 suppliers
$101.00/100mg
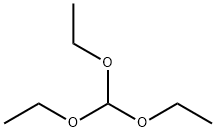
122-51-0
488 suppliers
$10.00/5ml
![7-CHLOROPYRIDO[3,2-D]PYRIMIDIN-4(3H)-ONE](/CAS/20180601/GIF/897360-16-6.gif)
897360-16-6
26 suppliers
inquiry
Yield:897360-16-6 1.03 g
Reaction Conditions:
at 155; for 22 h;
Steps:
11.2 7-Chloropyrido[3,2-d]pyrimidin-4(1H)-one
A suspension of 3-amino-5-chloropicolinamide (1.1 g, 6.41 mmol) in triethyl orthoformate (15.99 mL, 96 mmol) was stirred at 155° C. for 22 hours. After cooling to RT, the yellow precipitate was collected by vacuum filtration and washed with hexanes to yield the title intermediate (1.03 g, 5.67 mmol) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ ppm 8.20 (s, 1H) 8.27 (d, J=2.35 Hz, 1H) 8.80 (d, J=2.25 Hz, 1H) 12.68 (br. s., 1H). LC/MS (ESI m/z=182 (M+H).
References:
US2014/213581,2014,A1 Location in patent:Paragraph 0628
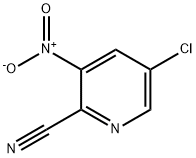
181123-11-5
173 suppliers
inquiry
![7-CHLOROPYRIDO[3,2-D]PYRIMIDIN-4(3H)-ONE](/CAS/20180601/GIF/897360-16-6.gif)
897360-16-6
26 suppliers
inquiry

27330-34-3
39 suppliers
$101.00/100mg
![7-CHLOROPYRIDO[3,2-D]PYRIMIDIN-4(3H)-ONE](/CAS/20180601/GIF/897360-16-6.gif)
897360-16-6
26 suppliers
inquiry