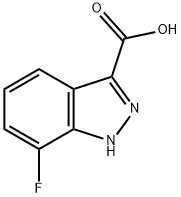
7-fluoro-1H-indazole-3-carboxylic acid synthesis
- Product Name:7-fluoro-1H-indazole-3-carboxylic acid
- CAS Number:959236-59-0
- Molecular formula:C8H5FN2O2
- Molecular Weight:180.14
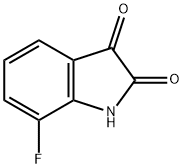
317-20-4
293 suppliers
$6.00/1g

959236-59-0
98 suppliers
$83.00/250mg
Yield: 47%
Reaction Conditions:
Stage #1:7-fluoro-1H-indole-2,3-dione with sodium hydroxide;sodium nitrite in waterCooling with ice;
Stage #2: with sulfuric acid in water at 0; for 0.166667 h;
Stage #3: with hydrogenchloride;tin(II) chloride dihdyrate in water at 0; for 1 h;
Steps:
10.1
This compound was prepared following the procedure of Johnson, B. L.; Rodgers, J. D. Syn. Comm. 2005, 35, 2681-2684. A suspension of 5.28 g 7-fluoroisatin in 30 mL of water was added 1.30 g NaOH, in 10 mL water with stirring. The resulting dark red solution was stirred until all of the solids dissolved and was then cooled in an ice water bath. The solution was then slowly added a cooled (ice bath) solution of 2.21 g NaNO2 in 10 mL water. These combined solutions were then added slowly to cooled (ice bath) to solution of aqueous sulfuric acid (3.4 mL H2SO4 in 60 mL water). Ice was added to maintain a temperature of approximately 0° C. After stirring for approximately 10 minutes, this dark red diazonium solution was added slowly to a chilled (0° C., ice bath) solution of 18 g SnCl22H2O in 30 mL concentrated HCl. Ice was again added to maintain a temperature of approximately 0° C. After stirring for approximately 1 hour, the reaction was filtered and the resulting residue was dissolved in 1 N NaOH (60 mL), washed with ether (2×50 mL). The resulting yellow-brown solution was cooled in an ice bath and acidified to a pH3 (litmus paper) with concentrated HCl, which resulted in the formation of a dark yellow precipitate. The precipitate was collected by filtration, washed with water, and dried over night in an oven to give 3.69 g (47%) of 7-fluoro-1H-indazole-3-carboxylic acid as an orange solid. 1H NMR (400 MHz, DMSO-d6) δ 14.35 (br s, 1H), 13.22 (br s, 1H), 7.89-7.87 (m, 1H), 7.26-7.21 (m, 2H). MS (ESI) m/z 181 (M+H)+.
References:
Pfizer Inc. US2011/28447, 2011, A1 Location in patent:Page/Page column 40