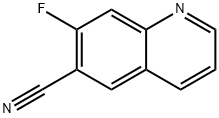
7-fluoroquinoline-6-carbonitrile synthesis
- Product Name:7-fluoroquinoline-6-carbonitrile
- CAS Number:956901-17-0
- Molecular formula:C10H5FN2
- Molecular Weight:172.16
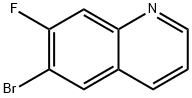
127827-52-5
103 suppliers
$18.00/250mg
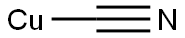
544-92-3
247 suppliers
$9.00/1g

956901-17-0
14 suppliers
inquiry
Yield:956901-17-0 47.6%
Reaction Conditions:
tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 120; for 12 h;
Steps:
32.2
Step 2: A suspension of compound B (25 g, 0.11 mol) and CuCN (12 g, 0.14 mol) in DMF (400 mL) was degassed by passing through N2, then Pd(PPh3)4 (6.5 g, 0.0056 mol) was added. The reaction mixture was heated to 120° C. for 12 h. The mixture was allowed to cool to room temperature and DMF was evaporated in vacuum. The residue was poured into water (100 mL) and extracted with CH2Cl2 (1000 mL×2). The combined organic phases were evaporated and the residue was dried in vacuo to afford crude compound C, which was purified by column chromatography (silica gel, EtOAc/Petroleum ether=1:15) to yield compound C (9 g, 47.6%) as a yellow solid.1H NMR (400 MHz, MeOH): δ 9.014 (dd, 1H), 8.664 (d, 1H), 8.495 (d, 1H), 7.981 (d, 1H), 7.626 (dd, 1H).
References:
US2007/265272,2007,A1 Location in patent:Page/Page column 31; 32
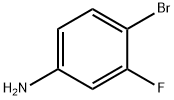
656-65-5
301 suppliers
$5.00/1g

956901-17-0
14 suppliers
inquiry
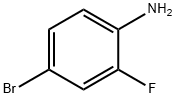
367-24-8
488 suppliers
$15.00/25g

956901-17-0
14 suppliers
inquiry