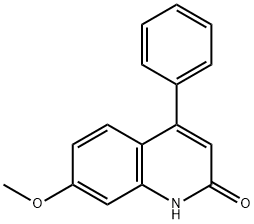
7-METHOXY-4-PHENYL-QUINOLIN-2-OL synthesis
- Product Name:7-METHOXY-4-PHENYL-QUINOLIN-2-OL
- CAS Number:30034-43-6
- Molecular formula:C16H13NO2
- Molecular Weight:251.28
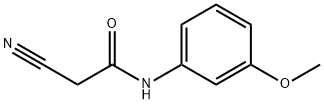
23058-90-4
7 suppliers
inquiry

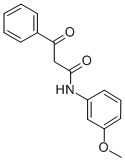
30034-43-6
12 suppliers
inquiry
Yield:30034-43-6 40 mg
Reaction Conditions:
with phosphoric acid in water at 100;
Steps:
General procedure for synthesis of β-ketoamides (4a-4d):
General procedure: In oven dried 5 mL vial contains the mixture of 2-cyano-(N-tolyl)-acetamide (30 mg, 0.17 mmol, 1.0 equiv.) arylboronic acid (2 equiv.), bipyridine (3 mg, 10 mol%), Pd(OAc)2 (5 mg. 10 mol%) were dissolved in THF solvent (2 mL). To this stirred solution, TFA (61 μl, 4 equiv.) was added and the reaction mixture was stirred vigorously at 80 oC for 22-30 hours. After complete consumption of the starting material (monitored by TLC), the reaction mixture was cooled to room temperature. Then the contents were poured into a separating funnel (25 mL) containing 5 mL of water. The work-up procedure was followed similar to the synthesis of 3a-3c. The crude compound was purified by flash column chromatography (100-200 Mesh size silica gel). Compound will elute in 20% EtOAc/Hexane (Rf = 0.6).
References:
Krishna Reddy, Singarajanahalli Mundarinti;Prasanna Kumari, Subramaniyan;Selva Ganesan, Subramaniapillai [Tetrahedron Letters,2021,vol. 79,art. no. 153296]