7-METHOXYINDOLE-3-ACETONITRILE synthesis
- Product Name:7-METHOXYINDOLE-3-ACETONITRILE
- CAS Number:2436-18-2
- Molecular formula:C11H10N2O
- Molecular Weight:186.21
Yield:2436-18-2 0.90 g
Reaction Conditions:
Stage #1: (7-methoxy-1H-indol-3-yl)-N,N-dimethylmethanaminewith methyl iodide in dichloromethane;toluene; for 12 h;
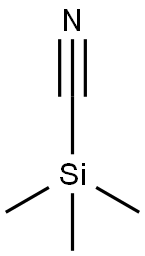
Stage #2: trimethylsilyl cyanidewith tetrabutyl ammonium fluoride in tetrahydrofuran; for 4 h;
Steps:
7e (7-methoxy-1H-indol-3-yl)-acetonitrile
General procedure: Following a similar procedure as the one detailedfor 7d, to a solution ofdimethylamine (40% aqueous, 1.2 mL, 10.9 mmol, 1.6 eq.) and formalin (37%aqueous, 720 μL, 8.9 mmol, 1.3 eq.) inglacial acetic acid (2 mL) and water (1 mL) was added 7-methoxyindole (1.0 g,6.8 mmol, 1.0 eq.). To the 7-methoxygramineobtained after work-up were added CH2Cl2 (20 mL), toluene(40 mL) and methyl iodide (845 mL, 13.6 mmol, 2.0eq.). The reaction mixture was stirred for 12 hours, concentrated to dryness, theresidue solubilized in THF (60 mL), then TMSCN (1.3 mL, 10.2 mmol, 1.5 eq.) andTBAF (20 mL, 1M, 20.4 mmol, 3.0 eq.) were added. The reaction mixture was stirredfor 4 hours and worked-up. Silicagel flash-column chromatography of the residue (elution with heptane/EtOAc,80/20 to 40/60) afforded 0.90 g of 7e (71 %) as an amorphous beige solid. IR νmax (cm-1): 2258 (ν CN),3369 (ν N-H). 1H RMN (d6-DMSO,300 MHz) d (ppm) : 3.91 (3H, s, 7-CH3O), 4.00 (2H, s, CH2),6.71 (1H, d, J6-5 = 7.7 Hz, H6), 6.99 (1H, t, J5-4 =J5-6 = 7.7 Hz, H5), 7.17 (1H, d, J4-5 = 7.7 Hz,H4), 7.23 (1H, s, H2), 11.22 (1H, s, H indolic). 13C RMN (d6-DMSO,75.5 MHz) d (ppm):13.8 (CH2), 55.6 (7-CH3O), 102.6 (C6), 104.6 (C3), 111.2(C4), 119.9 (C nitrile), 120.1 (C5), 124.0 (C2), 126.8 (C3a), 128.0 (C7a),146.7 (C7). ESI-MS: m/z 209.1 ([M+Na]+), 241.1 ([M+Na+MeOH]+). HRESI-MS: m/z 209.0685 (calcdfor C11H10N2ONa+209.0691).
References:
Labrière, Christophe;Talapatra, Sandeep K.;Thoret, Sylviane;Bougeret, Cécile;Kozielski, Frank;Guillou, Catherine [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 4,p. 721 - 734] Location in patent:supporting information
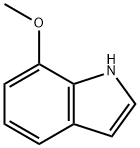
3189-22-8
264 suppliers
$16.00/1g

2436-18-2
24 suppliers
inquiry
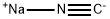
773837-37-9
0 suppliers
inquiry
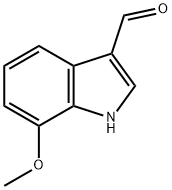
109021-59-2
81 suppliers
$65.00/250mg

2436-18-2
24 suppliers
inquiry