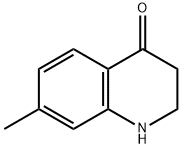
7-METHYL-2,3-DIHYDROQUINOLIN-4(1H)-ONE synthesis
- Product Name:7-METHYL-2,3-DIHYDROQUINOLIN-4(1H)-ONE
- CAS Number:36053-96-0
- Molecular formula:C10H11NO
- Molecular Weight:161.2
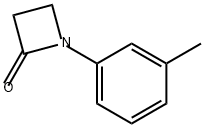
38560-26-8
0 suppliers
inquiry

36053-96-0
16 suppliers
$240.00/100mg
Yield:36053-96-0 46%
Reaction Conditions:
with trifluorormethanesulfonic acid in dichloromethane at 0 - 20; for 1 h;Inert atmosphere;
Steps:
50.3 Step 3: 7-methyl-2,3-dihydroquinolin-4(1H)-one (336-4)
To a solution of Compound 336-3 (5 g, 31.0 mmol) in DCM (100 mL) was added TfOH (9.3 g, 62.0 mmol) at 0°C under N2.The mixture was stirred at 20°C for 1hr. TLC indicated the reaction completed. The reaction mixture was quenched by poured into ice water (60 mL) and extracted with EtOAc (10 mL x 2). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue, which was purified by prep-HPLC to give Compound 336-7 (2.3 g, 14.3 mmol, 46% yield) as a yellow solid.1H NMR (400MHz, CHLOROFORM-d) d = 7.76- 7.74 (d, J=8 Hz, 1H), 6.58- 6.56 (d, J=8.4 Hz, 1H), 6.47 (s, 1H), 4.32 (s, 1H), 3.58 - 3.54 (m, 2H), 2.69- 2.66 (m, 2H), 2.26 (s, 3H).
References:
WO2021/3295,2021,A1 Location in patent:Paragraph 0547
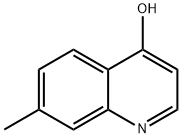
82121-08-2
47 suppliers
$65.00/100mg

36053-96-0
16 suppliers
$240.00/100mg

76227-95-7
2 suppliers
inquiry

36053-96-0
16 suppliers
$240.00/100mg
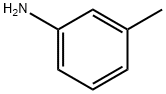
108-44-1
374 suppliers
$9.00/1g

36053-96-0
16 suppliers
$240.00/100mg

38560-21-3
3 suppliers
inquiry

36053-96-0
16 suppliers
$240.00/100mg