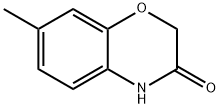
7-Methyl-2,4-dihydro-1,4-benzoxazin-3-one synthesis
- Product Name:7-Methyl-2,4-dihydro-1,4-benzoxazin-3-one
- CAS Number:39522-25-3
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
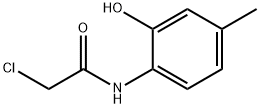
153505-95-4
0 suppliers
inquiry

39522-25-3
36 suppliers
$65.00/100mg
Yield:39522-25-3 127.9 kg
Reaction Conditions:
with 2,6-di-tert-butyl-4-methyl-phenol;potassium carbonate in tetrahydrofuran at 60 - 65; for 18 h;Industrial scale;
Steps:
1.ib
In a separate 4500 L reactor (named here R105) a suspension of potassium carbonate powder (134.7 kg, 975 mol, 1.2 eq) and tetrahydrofuran (217 kg, 2.5 eq vol) were stirred at 20 °C. The contents of reactor R103 were transferred to reactor R105 over 90 minutes maintaining the temperature at 20 to 25 °C. The reactor R103 and the transfer lines were rinsed with THF (50 kg). The contents of reactor R105 were warmed to 60 to 65 °C over 4 hours. After maintaining at 60 to 65 °C for 11 hours the suspension was sampled for analysis. HPLC showed 1.7 % 2-chloro-N-(2-hydroxy-4-methylphenyl)acetamide 3 remaining. The reactor contents were heated at 65 to 66 °C for a further 4 hours and re sampled. HPLC showed 99.9 % conversion of 2-chloro-N-(2-hydroxy-4- methylphenyl)acetamide 3. Demineralised water (1200 kg, 12 eq vol) was charged to reactor R105 over 90 minutes maintaining the temperature at 50 to 60 °C. Tetrahydrofuran was then distilled out under reduced pressure, initially at 600 mbar and pulling in to 50 mbar at the end of the strip (maximum jacket temperature 60 °C), distillate weight 1450 kg. The resulting aqueous suspension of the product was cooled to 20 to 25 °C, vented to nitrogen and stirred for 1 hour prior to filtration. The product was filtered off using a large Hastelloy pressure filter and blown down with nitrogen (filtrate weight 1228 kg). The light brown coloured solid was washed via the reactor with demineralised water (3 x 150 kg), blowing down each time (filtrate weight 440 kg).The damp filter-cake (146 kg) was dried in the double cone vacuum drier at 50 to 60 °C under reduced pressure to constant weight (discharge spec 0.1 % by KF) to give 7- methyl-2H-benzo[b][l,4]oxazin-3(4H)-one 4 as a light brown solid. Recorded product weight was 127.9 kg, yield (based on 'H NMR assay) = 93.9 %. The HPLC purity was 99.0 %, the 'll NMR assay was 97.3 % w/w. The water content, measured by the Karl- Fisher (KF) analysis, was 0.04 %. The procedure was repeated on 98 kg 2-Amino-5~methylphenol 1 and yielded 126.7 kg 7-methyl-2H-benzo[b][l,4]oxazin-3(4H)-one 4.
References:
WO2019/129588,2019,A1 Location in patent:Page/Page column 23; 24

2835-98-5
255 suppliers
$8.00/5g

79-04-9
381 suppliers
$12.00/5g

39522-25-3
36 suppliers
$65.00/100mg
139502-99-1
0 suppliers
inquiry

700-38-9
195 suppliers
$6.00/5g

105-36-2
352 suppliers
$5.00/5G

39522-25-3
36 suppliers
$65.00/100mg
139502-97-9
0 suppliers
inquiry
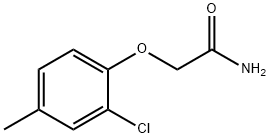
1248651-95-7
4 suppliers
inquiry

39522-25-3
36 suppliers
$65.00/100mg
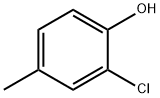
6640-27-3
164 suppliers
$10.00/1g

79-07-2
441 suppliers
$5.00/25g

39522-25-3
36 suppliers
$65.00/100mg