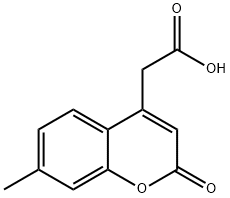
(7-METHYL-2-OXO-2H-CHROMEN-4-YL)ACETIC ACID synthesis
- Product Name:(7-METHYL-2-OXO-2H-CHROMEN-4-YL)ACETIC ACID
- CAS Number:50402-83-0
- Molecular formula:C12H10O4
- Molecular Weight:218.21
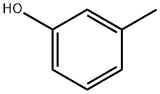
108-39-4
467 suppliers
$13.00/25g
77-92-9
1625 suppliers
$5.00/10g

50402-83-0
19 suppliers
$55.00/500mg
Yield:50402-83-0 88%
Reaction Conditions:
with sulfuric acid for 0.0666667 h;Microwave irradiation;
Steps:
Synthesis of coumarin-4-acetic acid analogs (2a-2f)
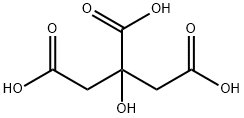
General procedure: Coumarin-4-acetic acid is a known compound (Manwaret al. 2008). The reaction conditions reported for thiscompound were modified and optimized (method A) tosynthesis the analogs 2a-2f. These analogs were also synthesizedby microwave assisted method (method B). Method A A mixture of citric acid (0.02 mol) and sulfuricacid (0.03 mol) was stirred at room temperature for 30 min.The mixture was kept in boiling water bath to removecarbon monoxide (CAUTION: Fume Cupboard). As soonas the evolution of CO gas was slackened, the flask wasremoved from the bath, kept aside for 15 min or till thereaction mixture was free from CO bubbles. The contentswere cooled to 10 °C, and (un)substituted phenol (0.02 mol)cooled to 10 °C was added drop wise. The reaction mixturewas stirred at room temperature for 48 h, and poured overcrushed ice. The precipitates were filtered and dissolved insaturated sodium bicarbonate solution. The solution wasacidified to afford the intermediates 2a-2f. Method B A mixture of citric acid (0.02 mol), sulphuricacid (0.03 mol) and (un)substituted phenol (0.02 mol) washeated in a microwave oven at 10% power for 4 min. Thereaction mixture was poured over crushed ice and processedsimilarly as in method A to obtain the desired product.
References:
Sethi, Purva;Bansal, Yogita;Bansal, Gulshan [Medicinal Chemistry Research,2018,vol. 27,# 1,p. 61 - 71]
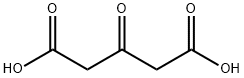
542-05-2
475 suppliers
$6.00/10g

108-39-4
467 suppliers
$13.00/25g

50402-83-0
19 suppliers
$55.00/500mg

77-92-9
1625 suppliers
$5.00/10g

50402-83-0
19 suppliers
$55.00/500mg

108-39-4
467 suppliers
$13.00/25g

105-50-0
394 suppliers
$6.00/5g

50402-83-0
19 suppliers
$55.00/500mg