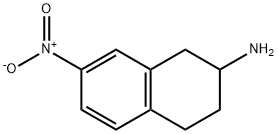
7-NITRO-1,2,3,4-TETRAHYDRO-NAPHTHALEN-2-YLAMINE synthesis
- Product Name:7-NITRO-1,2,3,4-TETRAHYDRO-NAPHTHALEN-2-YLAMINE
- CAS Number:101167-13-9
- Molecular formula:C10H12N2O2
- Molecular Weight:192.21
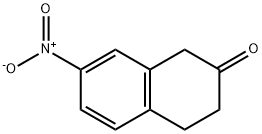
122520-12-1
30 suppliers
inquiry

101167-13-9
29 suppliers
inquiry
Yield: 65%
Reaction Conditions:
with ammonium acetate;sodium cyanoborohydride in methanol at 50; for 15 h;
Steps:
174.D
Step D: 7-Nitro-1,2,3,4-tetrahydro-2-naphthalenamine; 7-Nitro-3,4-dihydro-2(1H)-naphthalenone (200 mg, 1.1 mmol) was combined with ammonium acetate (807 mg, 10.5 mmol), sodium cyanoborohydride (86 mg, 1.4 mmol) and MeOH (5 mL) and heated to 50° C. for 15 h. The reaction was cooled and concentrated under vacuum and the residue was partitioned between EtOAc and water. The organic layer was washed with 5% aqueous potassium carbonate, brine and dried over Na2SO4. The crude was concentrated under vacuum and purified by silica gel flash column chromatography (0-100% (90% DCM/9% MeOH/1% NH4OH)/DCM). Purification provided 130 mg (65%) of the desired amine as a brown oil. 1H NMR (400 MHz, DMSO-d6) δ 7.90-7.89 (m, 2H), 7.31 (d, J=8.5 Hz, 1H), 3.06-2.88 (m, 3H), 2.84-2.72 (m, 1H), 2.54-2.44 (m, 2H), 1.81 (d, J=35.2 Hz, 2H), 1.51-1.42 (m, 1H).
References:
SmithKline Beecham Corporation US2008/51395, 2008, A1 Location in patent:Page/Page column 105
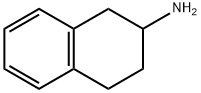
2954-50-9
89 suppliers
$50.00/50mg

101167-13-9
29 suppliers
inquiry