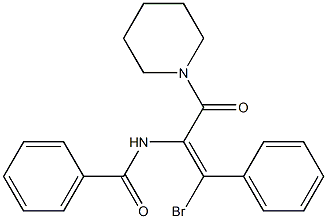
(Z)-N-(1-bromo-3-oxo-1-phenyl-3-(piperidin-1-yl)prop-1-en-2-yl)benzamide synthesis
- Product Name:(Z)-N-(1-bromo-3-oxo-1-phenyl-3-(piperidin-1-yl)prop-1-en-2-yl)benzamide
- CAS Number:705293-97-6
- Molecular formula:C21H21BrN2O2
- Molecular Weight:413.3076
Yield:705293-97-6 49%
Reaction Conditions:
Stage #1: piperidine;(4Z)-4-benzylidene-2-phenyl-1,3-oxazol-5(4H)-one in chloroform at 0; for 1 h;
Stage #2: with bromine;calcium carbonate in chloroform;
Steps:
2 Example 2; 1-[(Z)-1-OXO-2-(BENZOYLAMINO)-3-PHENYL-3-BROMOPROPENYLY PIPERIDINE.
To a solution of (1) (10.0 g, 40.1 mmol) in chloroform (200 mL) at 0 C was added dropwise a solution of piperidine (4.2 g, 49 mmol) in chloroform (60 mL). The yellow solution was stirred at 0 C for 1 hour. Solid calcium carbonate (6.0 g, 50 mmol) was added, followed by dropwise addition of bromine (6.4 g, 40 mmol) in chloroform (60 mL). The suspension was filtered to remove calcium salts, and the supernatant liquid was evaporated to dryness. The resulting orange oil was crystallized from ethanol, and further purified by recrystallization from ethanol/water. 8.18 g (49%) of pale green crystals were obtained, 158.2-158. 5 C.
References:
WO2004/50613,2004,A2 Location in patent:Page 56

842-74-0
11 suppliers
inquiry

705293-97-6
4 suppliers
inquiry

100-52-7
942 suppliers
$20.37/250g

705293-97-6
4 suppliers
inquiry

