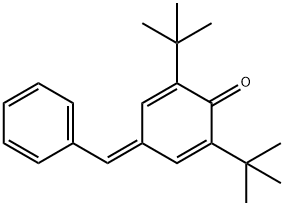
2,6-BIS(1,1-DIMETHYLETHYL)-4-(PHENYLMETHYLENE)-2,5-CYCLOHEXADIEN-1-ONE synthesis
- Product Name:2,6-BIS(1,1-DIMETHYLETHYL)-4-(PHENYLMETHYLENE)-2,5-CYCLOHEXADIEN-1-ONE
- CAS Number:7078-98-0
- Molecular formula:C21H26O
- Molecular Weight:294.43
Yield:7078-98-0 87%
Reaction Conditions:
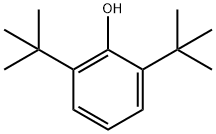
Stage #1: 2,6-di-t-butylphenol;benzaldehydewith pyrrolidine in methanol at 25; for 12 h;
Stage #2: in methanol;Petroleum ether at 90; for 0.166667 h;Solvent;
Steps:
1-3 One-step method for synthesizing 2,6-disubstituted-4-arylmethylene-2,5-cyclohexadien-1-one, the steps are as follows:
(1) In a 2-liter three-necked flask equipped with an electric stirring device, add 206.3 g (1.0 mol) of 2,6-di-tert-butylphenol, 112 mL (116.7 g, 1.1 mol) of benzaldehyde and 83 mL (71.1 mol) of pyrrolidine g, 1.0 mol), then add 1000 ml of methanol, stir the reaction at 25 ° C and track the reaction progress by thin layer chromatography; when 2,6-di-tert-butylphenol is completely consumed, stop the reaction (about 12 hours), often The methanol and pyrrolidine are recovered by pressure distillation to obtain the waxy solid of the crude product;(2) Dissolve the waxy solid of the crude product in 1500 mL of petroleum ether under heating and refluxing conditions at 90°C, heat under reflux for 10 minutes, naturally cool to room temperature, and let stand at room temperature to slowly separate out; if necessary, repeat the above After the operation, 257 g of recrystallized pure product was finally obtained (yield 87%, content 98.7%).
References:
CN113979846,2022,A Location in patent:Paragraph 0050-0061; 0079-0090
17330-09-5
4 suppliers
inquiry

7078-98-0
67 suppliers
inquiry
20017-39-4
0 suppliers
inquiry

7078-98-0
67 suppliers
inquiry

16939-57-4
41 suppliers
$45.00/50mg

7078-98-0
67 suppliers
inquiry