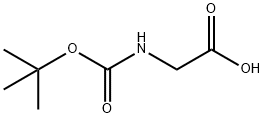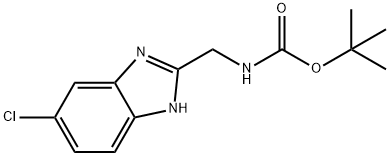
(6-Chloro-1H-benzoimidazol-2-ylmethyl)-carbamic acid tert-butyl ester synthesis
- Product Name:(6-Chloro-1H-benzoimidazol-2-ylmethyl)-carbamic acid tert-butyl ester
- CAS Number:712275-17-7
- Molecular formula:C13H16ClN3O2
- Molecular Weight:281.74
Yield:712275-17-7 79.1%
Reaction Conditions:
Stage #1: BOC-glycinewith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20; for 0.166667 h;
Stage #2: 4-Chloro-1,2-phenylenediamine in dichloromethane;N,N-dimethyl-formamide at 20;
Stage #3: with acetic acid at 70; for 2 h;
Steps:
83
A mixture of 2-((tert-butoxycarbonyl)amino)acetic acid (2.62 g, 15.0 mmol, 1.0 eq), EDC.HCl (3.44 g, 18.0 mmol, 1.2 eq), HOBt (2.83 g, 21.0 mmol, 1.4 eq) and DCM (150 mL) was stirred at rt for 10 min. Then 4- chlorobenzene-l,2-diamine (2.13 g, 15.0 mmol, 1.0 eq) and DMF(2 mL) were added, and the resulting mixture was stirred at rt overnight. DCM was evaporated and EA (150 mL) was added. The organic phase was washed with brine (30 mL), sat. NH4C1 (30 mL x 2), sat. NaHCOs (30 mL x 2) and finally with brine (30 mL). The resulting organic layer was dried with Na2S04, concentrated to dryness. Then 20 mL of AcOH was added and the mixture was heated to 70 °C and stirred for 2 h. After cooled to rt, the mixture was concentrated in vacuo to give a solid, purified by flash chromatography to afford tert-butyl ((6-chloro-lH-benzo[d]imidazol- 2-yl)methyl)c
References:
WO2015/103317,2015,A1 Location in patent:Page/Page column 163; 164

24424-99-5
825 suppliers
$13.50/25G
![(6-CHLORO-1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/273399-95-4.gif)
273399-95-4
50 suppliers
$45.00/250mg

712275-17-7
12 suppliers
inquiry

95-83-0
289 suppliers
$14.00/5g

712275-17-7
12 suppliers
inquiry